Microvascular Rarefaction in the Sinoatrial Node: A Potential Mechanism for Pacemaker Dysfunction in Early HFpEF
- PMID: 40704959
- PMCID: PMC12471107
- DOI: 10.1016/j.jacep.2025.06.007
Microvascular Rarefaction in the Sinoatrial Node: A Potential Mechanism for Pacemaker Dysfunction in Early HFpEF
Abstract
Background: Microvascular rarefaction is a feature of heart failure with preserved ejection fraction (HFpEF) that may underlie associated rhythm disturbances. Angiotensin II (AngII) signaling has been implicated, but its role in sinoatrial (SA) node dysfunction remains unclear.
Objectives: The authors tested whether changes in SA node microvascular architecture contribute to pacemaker dysfunction in early HFpEF.
Methods: Mice received a 28-day subcutaneous infusion of a sub-pressor dose of AngII. Electrocardiography, echocardiography, confocal imaging, spatial RNA detection, and optical mapping were used to assess SA node structure and function.
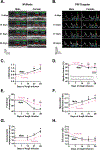
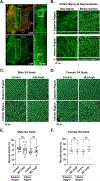
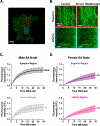
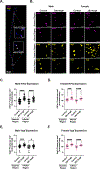
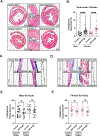
Results: Heart rate declined progressively during AngII infusion, with males falling from 605 ± 6 beats/min to 490 ± 6 beats/min and females from 646 ± 23 beats/min to 511 ± 10 beats/min by day 28. Bradycardia was accompanied by increased beat-to-beat variability: the percentage of consecutive heartbeats that differed in duration by >6 milliseconds increased from 3.5% ± 1.3% to 32.1% ± 4.5% in males and from 3.8% ± 1.1% to 27.7% ± 2.5% in females. These changes coincided with reduced microvessel density in the superior SA node (males: 6.1 ± 0.5 nm/μm3 to 3.9 ± 0.2 nm/μm3; females: 5.6 ± 0.4 to 2.8 ± 0.5 nm/μm3), whereas vessels in the inferior SA node remained unchanged. Despite preserved myocyte density, these changes were accompanied by up-regulation of oxidative stress and the hypoxia-inducible factor 1α and vascular endothelial growth factor signaling pathways.
Conclusions: These findings highlight microvascular rarefaction in the superior SA node as a key early event in HFpEF pathology. The loss of redundant vascular loops compromises metabolic support for pacemaking, illustrating a broader principle: rarefaction can impair excitability in metabolically demanding excitable tissues.
Keywords: HFpEF; sinoatrial node; vascular rarefaction.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures The project was supported by National Institutes of Health grants HL168874 (Dr Santana), TR001860 (TL1 TR001861) (Dr Rivera), HL163930 (Dr Contreras), UL1 TR001860 (Dr Bautista), TR001859 (Dr Bautista), GM130459 (Dr Grainger), HL085727 (Dr Chiamvimonvat), HL085844 (Dr Chiamvimonvat), HL170520 (Dr Chiamvimonvat), HL160274 (Dr Chiamvimonvat), and OD010389. The work was further supported by American Heart Association grants 25POST1378853 (Dr Rhana), 24CDA1276831 (Dr Thai), 23SFRNCCS1052478 (Dr Chiamvimonvat), 23SFRNPCS1060482 (Dr Chiamvimonvat), as well as a Harold S. Geneen Charitable Trust Awards for Coronary Heart Disease (Dr Thai). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures






References
-
- DiFrancesco D Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature 1986;324:470–3. - PubMed
-
- Bogdanov KY, Maltsev VA, Vinogradova TM et al. Membrane potential fluctuations resulting from submembrane Ca2+ releases in rabbit sinoatrial nodal cells impart an exponential phase to the late diastolic depolarization that controls their chronotropic state. Circ Res 2006;99:979–87. - PubMed
-
- Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol 2018;15:457–470. - PubMed

