Impact of Neoadjuvant Chemotherapy on Surgical Outcomes and Conversion to Node-Negativity in Invasive Lobular Breast Cancer: Analysis of Molecularly High-Risk Tumors by Histologic Subtype on the I-SPY2 Clinical Trial
- PMID: 40705266
- PMCID: PMC12494636
- DOI: 10.1245/s10434-025-17862-0
Impact of Neoadjuvant Chemotherapy on Surgical Outcomes and Conversion to Node-Negativity in Invasive Lobular Breast Cancer: Analysis of Molecularly High-Risk Tumors by Histologic Subtype on the I-SPY2 Clinical Trial
Abstract
Background: Invasive lobular carcinoma (ILC) has lower response rates to neoadjuvant chemotherapy (NAC) than invasive ductal carcinoma. While ILC often has low-risk biology, there is a high-risk subset within this heterogeneous tumor type. We compared surgical treatment and response rates by histology in I-SPY2, a multicenter NAC trial.
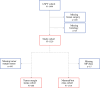
Methods: We evaluated 1329 patients with stage II-III breast cancer and high-risk 70-gene assay. Patients with classic, pleomorphic, or mixed lobular/ductal histology were included in the lobular cohort. We evaluated rates of mastectomy, positive margins, axillary dissection, and conversion from clinical node-positive (cN+) to pathologic node-negative (ypN-) status after NAC.
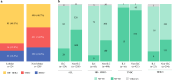
Results: Overall, 124 patients (9.3%) had lobular histology, with 69% being hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-). There was no difference in mastectomy rate (57.2% for lobular vs. 55.8% for non-lobular). The ILC cohort had more positive margins after lumpectomy than the non-ILC cohort (21.2% vs. 7.9%; p = 0.023). Within cN0 cases, axillary dissection was significantly more common among the lobular cases (24.1% vs. 14.0%; p = 0.039). Conversion from cN+ to ypN0 did not differ statistically between lobular and non-lobular cases (40.9% vs. 51.2%; p = 0.11). The nodal conversion rate among cN+lobular tumors was 30.6% in HR+/HER2-, 72.7% in HER2+, and 66.7% in triple-negative cases.
Conclusions: These data demonstrate the challenges of surgical management for ILC but hold promise that molecular classification can improve treatment selection. While high genomic risk is generally less common among ILC, our findings suggest that gene expression assays in cN+ILC patients can identify a subset who may benefit from NAC.
Keywords: Breast cancer; Lobular carcinoma; Neoadjuvant chemotherapy; Nodal response.
© 2025. The Author(s).
Conflict of interest statement
Disclosure: Rita A. Mukhtar reports research funding from GE Healthcare. A. Jo Chien reports institutional research funding from Merck, Amgen, Puma, Seagen, Pfizer, Olema; and advisory roles with AstraZeneca and Genentech. Kamran Ahmed reports institutional research funding from Eli Lilly, Genentech, and Gilead. Lily Gutnik reports research funding from the American Society of Clinical Oncology (ASCO) and the National Cancer Institute (NCI); and is an unpaid advisory board member for the Know Your Lemons Foundation and PretacPlus. M. Catherine Lee reports I-SPY2 trial funding, paid to her institution. Angela DeMichele reports institutional research funding from Novartis, Pfizer, Genentech, and Neogenomics; and serves as Program Chair for the Scientific Advisory Committee at ASCO. Douglas Yee reports research funding from National Institutes of Health (NIH)/NCI P30 CA 077598, P01 CA234228-01 and R01CA251600; consulting fees from Martell Diagnostics; and honoraria and travel for speaking at the International Breast Cancer Conference. Nola M. Hylton reports institutional research funding from the NIH. W. Fraser Symmans reports shares in IONIS Pharmaceuticals and Eiger Biopharmaceuticals; has received consulting fees from AstraZeneca; is a co-founder with equity in Delphi Diagnostics; and has issued patents for (1) a method to calculate residual cancer burden, and (2) genomic signature to measure sensitivity to endocrine therapy. Chantal Reyna reports Elucent and agenda consulting, terminated 2022. Hope S. Rugo reports institutional research support from AstraZeneca, Daiichi Sankyo, Inc., F. Hoffmann-La Roche AG/Genentech, Inc., Gilead Sciences, Inc., Lilly, Merck & Co., Novartis Pharmaceuticals Corporation, Pfizer, Stemline Therapeutics, OBI Pharma, Ambrx, and Greenwich Pharma; and advisory and consulting roles with Chugai, Puma, Sanofi, Napo, and Mylan. MD reports receiving research grants from the NIH/NCI and NIH/National Institute on Aging (NIA), and contracts from the Patient-Centered Outcomes Research Institute (PCORI). Rebecca Shatsky reports institutional research funding from OBI Pharmaceuticals, Quantum Leap Healthcare Collaborative, AstraZeneca and Gilead; serves on the AstraZeneca and Stemline Advisory Boards and the Gilead Speaker’s Bureau; has undertaken a consultancy role with Quantum Leap Healthcare Collaborative; and declares consulting/advisory roles for Novartis, Lilly, AstraZeneca, Daiichi Sankyo, Stemline, and Gilead. Claudine Isaacs reports institutional research funding from Tesaro/GSK, Seattle Genetics, Pfizer, AstraZeneca, BMS, Genentech, Novartis, and Regeneron; consultancy roles with AstraZeneca, Genentech, Gilead, ION, Merck, Medscape, MJH Holdings, Novartis, Pfizer, PUMA, Seagen; and royalties from Wolters Kluwer (UptoDate), and McGraw Hill (Goodman and Gillman). Laura J. Esserman reports participation on the Blue Cross Medical Advisory Panel; and is an uncompensated board member of Quantum Leap Healthcare Collaborative. Laura J. van 't Veer is a founding advisor and shareholder of Exai Bio; and part-time employee of, and owns stock in, Agendia. Judy C. Boughey receives institutional research funding from Eli Lilly, SimBioSys and Quantum Leap Healthcare; and serves on the Data Safety Monitoring Board for Cairn Surgical. Katrina Dimitroff, Christina Yau, Eileen P. Connolly, Marissa Howard-McNatt, Roshni Rao, Velle Ladores, Mehra Golshan, Candice A. Sauder, Rachael Lancaster, Jana Fox, Julia Tchou, Nicolas Prionas, Cletus A. Arciero, Henry Kuerer, Kayla Switalla, Neil Taunk, Todd M. Tuttle, Meena S. Moran, Lauren M. Postlewait, and Jane Perlmutter declare no competing interests that may be relevant to the contents of this study.
Figures
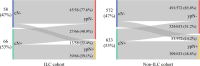

References
-
- Fayanju OM, Ren Y, Thomas SM, et al. The clinical significance of breast-only and node-only pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT): a review of 20,000 breast cancer patients in the national cancer data base (NCDB). Ann Surg. 2018;268(4):591–601. 10.1097/SLA.0000000000002953. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

