Cardiovascular Events in Individuals Treated With Sulfonylureas or Dipeptidyl Peptidase 4 Inhibitors
- PMID: 40705329
- PMCID: PMC12290728
- DOI: 10.1001/jamanetworkopen.2025.23067
Cardiovascular Events in Individuals Treated With Sulfonylureas or Dipeptidyl Peptidase 4 Inhibitors
Abstract
Importance: Sulfonylureas are commonly used to treat type 2 diabetes (T2D). Research findings on cardiovascular risk associated with sulfonylureas have been inconsistent.
Objective: To emulate a target trial that compares the risk of cardiovascular events after initiation of treatment with individual sulfonylureas or dipeptidyl peptidase 4 inhibitors (DPP4is).
Design, setting, and participants: This comparative effectiveness research study included individuals with T2D and moderate cardiovascular risk treated with metformin monotherapy who received care at 1 of 10 US health systems or were insured by 1 of 2 large health insurance plans between January 1, 2014, and January 1, 2023. Data were analyzed from July 2024 to March 2025.
Exposure: Initiation of treatment with a sulfonylurea (glimepiride, glipizide, or glyburide) or a DPP4i (reference category) as a second line therapy after metformin.
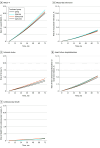
Main outcomes and measurements: The primary outcome was a 4-point composite of major adverse cardiovascular events (MACE-4): myocardial infarction, ischemic stroke, heart failure hospitalization, or cardiovascular death (from any of these conditions). The 5-year risks of each outcome were estimated.
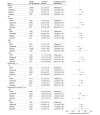
Results: Among 48 165 eligible individuals (median [IQR] age, 61 [52-69] years; 22 674 female [47.1%]; median [IQR] hemoglobin A1C, 7.8% [7.3%-8.5%]; median [IQR] low-density lipoprotein cholesterol, 89 mg/dL [70-112 mg/dL]), 18 147 started glipizide, 14 282 started glimepiride, 1887 started glyburide, and 13 849 started a DPP4i. Over the median (IQR) follow-up of 37 (20-64) months, 3158 individuals (6.6%) experienced a MACE-4. The estimated 5-year risks of MACE-4 were 8.1% (95% CI, 7.5%-8.7%) for DPP4i, 8.4% (95% CI, 6.8%-9.9%) for glyburide, 8.6% (95% CI, 7.9%-9.2%) for glimepiride, and 9.1% (95% CI, 8.7%-9.7%) for glipizide. Compared with DPP4is, the 5-year risk ratio of MACE-4 was 1.13 (95% CI, 1.03-1.23) for glipizide, 1.07 (95% CI, 0.96-1.16) for glimepiride, and 1.04 (95% CI, 0.83-1.24) for glyburide.
Conclusions and relevance: In this comparative effectiveness research study of sulfonylureas vs DPP4i in patients with T2D, the risk of MACE-4 events was highest for glipizide. These findings suggest that sulfonylureas, glipizide in particular, may not be the optimal agent in treatment of individuals with T2D at moderate cardiovascular risk.
Conflict of interest statement
Figures
Comment in
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

