Microbiota Transplantation Among Patients Receiving Long-Term Care: The Sentinel REACT Nonrandomized Clinical Trial
- PMID: 40705333
- PMCID: PMC12290730
- DOI: 10.1001/jamanetworkopen.2025.22740
Microbiota Transplantation Among Patients Receiving Long-Term Care: The Sentinel REACT Nonrandomized Clinical Trial
Abstract
Importance: Intestinal multidrug-resistant organism (MDRO) colonization is highly prevalent in long-term acute care hospital (LTACH) patients and is associated with MDRO infection and transmission. However, there are no therapies approved by the US Food and Drug Administration to reduce intestinal MDRO colonization.
Objective: To determine the safety and acceptability of fecal microbiota transplantation (FMT) in LTACH patients.
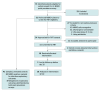
Design, setting, and participants: This single-center, open-label nonrandomized clinical trial was conducted from April to December 2023 at an LTACH in the Southeastern US with median 50-patient census and 28-day length of stay. Patients with MDRO colonization were identified by perirectal prevalence sampling. Patients colonized with at least 1 target MDRO were approached for informed consent for FMT. FMT recipients were compared with untreated controls with MDRO colonization. Data were analyzed from August 2024 to May 2025.
Intervention: Healthy donor fecal microbiota (50-100 g stool and 250 mL normal saline with 9% glycerol) instilled via gastrostomy tube or enema without antibiotic or bowel preparation conditioning.
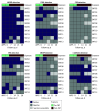
Main outcomes and measures: The primary outcome was frequency and severity of adverse events. Solicited adverse events were recorded for 7 days. Unsolicited adverse events were recorded for 6 months. Four weekly perirectal MDRO cultures were performed after FMT.
Results: A total of 42 patients, including 10 (mean [SD] age, 63.8 (14.5) years; 7 [70%] female) who received FMT and 32 contemporaneous controls (mean [SD] age, 64.0 [13.7] years; 13 [41%] female) were assessed. In 2 prevalence surveys, 23 of 32 (72%) and 26 of 34 (77%) perirectal cultures grew at least 1 MDRO. Among the FMT group, 5 patients received FMT via gastrostomy alone, 4 via enema alone, and 1 with both routes more than 30 days apart. No serious adverse events were attributed to FMT, and post-FMT solicited adverse events were mild. At final visit, all perirectal cultures from FMT recipients grew at least 1 MDRO. Post hoc analyses found numerically fewer FMT recipients had positive blood culture results (0 individuals vs 6 individuals [19%]; P = .31), pathogen intestinal dominance (2 of 8 individuals [25%] vs 4 of 8 individuals [50%]; P = .61), and 7 fewer days of antibiotic therapy per 1000 patient days (median [IQR], 12.6 [0-25.2] days vs 19.7 [6.5-36.1] days; P = .38) compared with controls in the 6 months after prevalence survey, although these differences were not statistically significant. Accounting for higher baseline FMT recipient antibiotic use, difference-in-differences analysis estimated 26 (95% CI, -64 to 12) fewer days of antibiotic therapy per 1000 patient-days after FMT, although this difference was also not statistically significant.
Conclusions and relevance: In this nonrandomized pilot clinical trial, FMT was acceptable for LTACH patients without related serious adverse events. Although not powered to test these outcomes, this study found potential reductions in bacteremia, intestinal pathogen domination, and antibiotic use associated with FMT, suggesting FMT should be evaluated in larger, randomized trials.
Trial registration: ClinicalTrials.gov Identifier: NCT05780801.
Conflict of interest statement
Figures

References
-
- Centers for Disease Control and Prevention.; National Center for Emerging Zoonotic and Infectious Diseases; National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention; National Center for Immunization and Respiratory Diseases . Antibiotic Resistance Threats in the United States, 2019. Centers for Disease Control and Prevention; 2019.
-
- Centers for Disease Control and Prevention; US Food and Drug Administration . Drug development considerations for the prevention of healthcare-associated infections. August 30, 2022. Accessed February 3, 2025. https://www.fda.gov/drugs/news-events-human-drugs/drug-development-consi...
-
- Shimasaki T, Seekatz A, Bassis C, et al. ; Centers for Disease Control and Prevention Epicenters Program . Increased relative abundance of Klebsiella pneumoniae carbapenemase–producing Klebsiella pneumoniae within the gut microbiota is associated with risk of bloodstream infection in long-term acute care hospital patients. Clin Infect Dis. 2019;68(12):2053-2059. doi: 10.1093/cid/ciy796 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

