Immunoreceptor CD300a regulates ischemic tissue damage and adverse remodeling in the mouse heart and kidney
- PMID: 40705472
- PMCID: PMC12483560
- DOI: 10.1172/JCI184984
Immunoreceptor CD300a regulates ischemic tissue damage and adverse remodeling in the mouse heart and kidney
Abstract
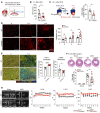
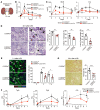
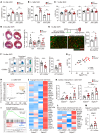
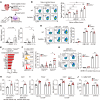
Acute ischemic organ diseases such as acute myocardial infarction and acute kidney injury often result in irreversible tissue damage and progress to chronic heart failure (CHF) and chronic kidney disease (CKD), respectively. However, the molecular mechanisms underlying the development of CHF and CKD remain incompletely understood. Here, we show that mice deficient in CD300a, an inhibitory immunoreceptor expressed on myeloid cells, showed enhanced efferocytosis by tissue-resident macrophages and decreased damage-associated molecular patterns and pathogenic SiglecFhi neutrophils, resulting in milder inflammation-associated tissue injury than in wild-type mice after ischemia and reperfusion (IR). Notably, we uncovered that CD300a deficiency on SiglecFlo neutrophils increased the signal transducer and activator of transcription 3-mediated production of pro-angiogenic and antifibrotic factors, resulting in milder adverse remodeling after IR. Our results demonstrated that CD300a plays an important role in the pathogenesis of ischemic tissue injury and adverse remodeling in the heart and kidney.
Keywords: Fibrosis; Immunology; Inflammation; Macrophages; Neutrophils.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

