Effect of obesity on the acute response to SARS-CoV-2 infection and development of post-acute sequelae of COVID-19 (PASC) in nonhuman primates
- PMID: 40705709
- PMCID: PMC12289017
- DOI: 10.1371/journal.ppat.1012988
Effect of obesity on the acute response to SARS-CoV-2 infection and development of post-acute sequelae of COVID-19 (PASC) in nonhuman primates
Abstract
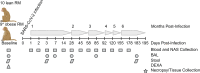
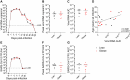
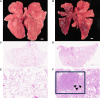
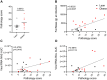
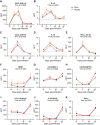
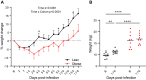
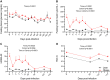
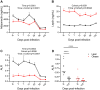
Long-term adverse consequences of SARS-CoV-2 infection, termed "long COVID" or post-acute sequelae of COVID (PASC), are a major component of overall COVID-19 disease burden. Prior obesity and metabolic disease increase the severity of acute disease, but SARS-CoV-2 infection also contributes to the development of new-onset metabolic disease. Since the COVID pandemic occurred in the context of the global obesity epidemic, an important question is the extent to which pre-existing obesity modifies long-term responses to SARS-CoV-2 infection. We utilized a nonhuman primate model to compare the effects of infection with the SARS-CoV-2 delta variant in lean and obese/insulin-resistant adult male rhesus macaques over a 6-month time course. While some longitudinal responses to SARS-CoV-2 infection, including overall viral dynamics, SARS-CoV-2-specific IgG induction, cytokine profiles, and tissue persistence of viral RNA, did not appreciably differ between lean and obese animals, other responses, including neutralizing Ab dynamics, lung pathology, body weight, degree of insulin sensitivity, adipocytokine profiles, body temperature, and nighttime activity levels were significantly different in lean versus obese animals. Furthermore, several parameters in lean animals were altered following SARS-CoV-2 infection to resemble those in obese animals. Notably, persistent changes in multiple parameters were present in most animals, suggesting that PASC may be more prevalent than estimated from self-reported symptoms in human studies.
Copyright: © 2025 Sauter et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: MKS has equity in Najit Technologies and serves as President and CSO. DNS has received research funding from Evrys Bio and Valneva USA. SEK is on the advisory boards for Amgen, Biomea Fusion, Eli Lilly, Merck, and Novo Nordisk, has equity in AltPep, has served as consultant for Neuroimmune and Oramed, and has received research funding from Corcept Therapeutics. JBS has equity in CytoDyn and has served as a consultant for Mabloc. PK has served as a consultant for Alnylam Pharmaceuticals, Courage Therapeutics, Crinetics Pharmaceuticals, and Junevity, and has received research funding from Novo Nordisk. CTR has equity in Diabetomics. All other authors have declared that no conflict of interest exists.
Figures


Update of
-
Effect of obesity on the acute response to SARS-CoV-2 infection and development of post-acute sequelae of COVID-19 (PASC) in nonhuman primates.bioRxiv [Preprint]. 2025 Feb 22:2025.02.18.638792. doi: 10.1101/2025.02.18.638792. bioRxiv. 2025. Update in: PLoS Pathog. 2025 Jul 24;21(7):e1012988. doi: 10.1371/journal.ppat.1012988. PMID: 40027795 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

