BBK32 attenuates antibody-dependent complement-mediated killing of infectious Borreliella burgdorferi isolates
- PMID: 40705830
- PMCID: PMC12316396
- DOI: 10.1371/journal.ppat.1013361
BBK32 attenuates antibody-dependent complement-mediated killing of infectious Borreliella burgdorferi isolates
Abstract
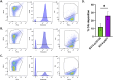
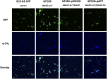
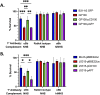
Borreliella burgdorferi, the causative agent of Lyme disease, has evolved unique complement evasion proteins that promote its ability to establish and maintain infection in mammalian hosts. Among these is B. burgdorferi BBK32, a multifunctional surface lipoprotein that binds extracellular matrix (ECM) components, including fibronectin (Fn). In addition to its ECM-binding functions, BBK32 binds to C1r, the initiator protease of the classical pathway of complement, and protects B. burgdorferi from complement-mediated killing following exposure to normal human serum. The disparate functions of BBK32 in adhesion and complement evasion have previously been studied in isolation. Herein we demonstrate that full-length BBK32 binds both Fn and C1 concurrently, indicating that binding of these macromolecules do not sterically hinder their simultaneous interaction. Given the link of antibody dependence to the classical pathway, we tested how the presence of BBK32 would protect infectious B. burgdorferi from borrelial-specific antibodies in a complement-dependent manner. BBK32 provided protection against complement activation in the presence of borrelial-specific antibodies in vitro. We also demonstrated, using both flow cytometry and fluorescence microscopy, that BBK32 results in the reduction of C4 deposition on the surface of borrelial cells. This work demonstrates that BBK32 can simultaneously bind to both C1r and Fn and contributes to the broader understanding of the ability of B. burgdorferi to evade antibody-dependent complement-mediated killing. These observations are significant as they suggest that BBK32 plays a dual role in adhesion and dissemination in infectious B. burgdorferi, as well as immune evasion activities, which ostensibly promotes its pathogenic potential.
Copyright: © 2025 Powell-Pierce et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

