De novo design and structure of a peptide-centric TCR mimic binding module
- PMID: 40705894
- PMCID: PMC12313176
- DOI: 10.1126/science.adv3813
De novo design and structure of a peptide-centric TCR mimic binding module
Abstract
T cell receptor (TCR) mimics offer a promising platform for tumor-specific targeting of peptide-major histocompatibility complex (pMHC) in cancer immunotherapy. In this study, we designed a de novo α-helical TCR mimic (TCRm) specific for the NY-ESO-1 peptide presented by human leukocyte antigen (HLA)-A*02, achieving high on-target specificity with nanomolar affinity (dissociation constant Kd = 9.5 nM). The structure of the TCRm-pMHC complex at 2.05-Å resolution revealed a rigid TCR-like docking mode with an unusual degree of focus on the up-facing NY-ESO-1 side chains, suggesting the potential for reduced off-target reactivity. Indeed, a structure-informed in silico screen of 14,363 HLA-A*02 peptides correctly predicted two off-target peptides, yet our TCRm maintained peptide selectivity and cytotoxicity as a T cell engager. These results represent a path for precision targeting of tumor antigens with peptide-focused α-helical TCR mimics.
Conflict of interest statement
Competing interests:
K.C.G. is a co-founder of 3T Biosciences and consults for Xaira Therapeutics. All other authors declare no competing interests.
Figures
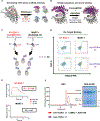
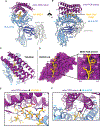


Update of
-
De novo design and structure of a peptide-centric TCR mimic binding module.bioRxiv [Preprint]. 2025 Apr 14:2024.12.16.628822. doi: 10.1101/2024.12.16.628822. bioRxiv. 2025. Update in: Science. 2025 Jul 24;389(6758):375-379. doi: 10.1126/science.adv3813. PMID: 39763827 Free PMC article. Updated. Preprint.
References
-
- Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J, T Cell Antigen Receptor Recognition of Antigen-Presenting Molecules. Annu. Rev. Immunol 33, 169–200 (2015). - PubMed
-
- Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA, Mechanisms of MHC class I‐restricted antigen processing and cross‐presentation. Immunological Reviews 207, 145–157 (2005). - PubMed
-
- Garcia KC, Adams EJ, How the T Cell Receptor Sees Antigen—A Structural View. Cell 122, 333–336 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

