Harnessing Facebook to Investigate Real-World Mentions of Adverse Events of Glucagon-Like Peptide-1 Receptor Agonist (GLP-1 RA) Medications: Observational Study of Facebook Posts From 2022 to 2024
- PMID: 40706081
- PMCID: PMC12289294
- DOI: 10.2196/73619
Harnessing Facebook to Investigate Real-World Mentions of Adverse Events of Glucagon-Like Peptide-1 Receptor Agonist (GLP-1 RA) Medications: Observational Study of Facebook Posts From 2022 to 2024
Abstract
Background: In recent years, there has been a dramatic increase in the popularity and use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) for weight loss. As such, it is essential to understand users' real-world discussions of short-term, long-term, and co-occurrent adverse events associated with currently used GLP-1 RA medications.
Objective: This study aims to quantitatively analyze temporal and co-occurrent GLP-1 RA adverse event trends through discussions of GLP-1 RA weight loss medications on Facebook from 2022 to 2024.
Methods: We collected 64,202 Facebook posts (59,293 posts after removing duplicate posts) from January 1, 2022, to May 31, 2024, through CrowdTangle, a public insights tool from Meta. Using English language social media posts from the United States, we examined discussions of adverse event mentions for posts referencing 7 GLP-1 RA weight loss product categories (ie, semaglutide, Ozempic, Wegovy, tirzepatide, Mounjaro, Zepbound, and GLP-1 RA as a class). All analyses were conducted using Python (version 3; Python Software Foundation) in a Google Colab environment.
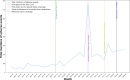
Results: Temporal time series analysis revealed that the GLP-1 RAs' adverse event mentions on social media aligned with several key events: the Food and Drug Administration's approval of Wegovy for pediatric weight management in December 2022, increased media coverage in August 2023, celebrity endorsement in December 2023, and Medicare Part D coverage expansion for weight loss medications in March 2024. Gastrointestinal (GI)-related adverse events (general term) were most prevalent for posts mentioning the GLP-1 RA class (210/4885, 4.30%) and Mounjaro (241/4031, 5.98%). In contrast, the most prevalent adverse event mentions noted for tirzepatide were headache (78/4202, 1.86%) and joint pain (71/4202, 1.69%). Hypertension (13/1769, 0.73%) was frequently mentioned in Zepbound posts, while pancreatitis was commonly associated with Mounjaro posts (44/4031, 1.08%), and 2.85% (139/4885) of posts broadly referring to the GLP-1 RA class. Furthermore, an integrated node network analysis revealed 3 distinct GLP-1 RA adverse events-mentioned clusters: cluster 1 (purple) contained allergies, anxiety, depression, chronic obstructive pulmonary disease, fatigue, fever, hypertension, indigestion, insomnia, gastroesophageal reflux disease, hives, swelling, restlessness, and seizures. Cluster 2 (pink) contained constipation, dehydration, headache, diarrhea, dizziness, hypoglycemia, sweating, and jaundice. Cluster 3 (brown) contained GI symptoms, such as nausea, pancreatitis, rash, and vomiting. The GI symptoms, such as nausea, vomiting, pancreatitis, diarrhea, and indigestion, were strongly associated together (≥100 co-occurrence mentions), while the mentioned neurological symptoms, such as anxiety, depression, and insomnia, were highly correlated with each other (50-100 co-occurrence mentions).
Conclusions: This social media study highlights the adverse event mention patterns for posts referencing GLP-1 RA medications. While further research is needed to rigorously examine and validate these findings, this study demonstrates the importance of monitoring social media discussions to predict novel, underreported, or rare drug adverse events, thereby improving patient care, clinical research, and health policy interventions.
Keywords: GLP-1 RAs; Mounjaro; Ozempic; Wegovy; Zepbound; adverse events; obesity management; semaglutide; social media; tirzepatide; weight loss medication.
© Amrutha S Alibilli, Vidur Jain, Heran Mane, Xiaohe Yue, Alexandria Ratzki-Leewing, Junaid S Merchant, Shaniece Criss, Quynh C Nguyen, Rozalina G McCoy, Thu T Nguyen. Originally published in JMIR Infodemiology (https://infodemiology.jmir.org).
Conflict of interest statement
Figures

References
-
- National diabetes statistics report. Centers for Disease Control and Prevention (Diabetes) 2024. [04-03-2025]. https://www.cdc.gov/diabetes/php/data-research/index.html URL. Accessed.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

