CDX2 expression as a predictive and prognostic biomarker of 5-FU response in cancer of unknown primary
- PMID: 40706223
- PMCID: PMC12309936
- DOI: 10.1016/j.esmoop.2025.105515
CDX2 expression as a predictive and prognostic biomarker of 5-FU response in cancer of unknown primary
Abstract
Background: Cancer of unknown primary (CUP) remains a diagnostic and therapeutic challenge with limited treatment guidance. Caudal type homeobox 2 (CDX2), a transcription factor indicative of intestinal differentiation, may define a distinct subset of CUP with potential sensitivity to 5-fluorouracil (5-FU)-based chemotherapy. This study examines outcomes in patients with CDX2-positive CUP and the predictive value of CDX2 expression for response to 5-FU-based regimens.
Patients and methods: We analyzed data from CDX2-positive CUP patients in the Mayo Clinic Cancer of Unknown Primary registry. All patients underwent comprehensive diagnostic evaluations and received first-line systemic therapy, categorized as 5-FU-based or non-5-FU-based. Primary endpoints were objective response rate (ORR) and clinical benefit rate; secondary endpoints included progression-free survival at first progression (PFS1) and overall survival. Logistic and Cox regression models were used to identify predictors of ORR and survival.
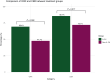
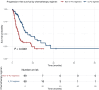
Results: A total of 209 CDX2-positive CUP patients met inclusion criteria. The median age was 64 years (range 18-89 years), with 115 (55%) female patients. Adenocarcinoma was the predominant histology in 147 patients (74.7%), while only 39 (18.7%) exhibited the classical colorectal-like immunoprofile (CK7-negative/CK20-positive/CDX2-positive). Most patients (203, 97.1%) had multiple metastatic sites, and 98 (46.9%) received 5-FU-based chemotherapy regimens. 5-FU-Based treatment was associated with a significantly higher ORR compared with non-5-FU regimens [68 of 98 (69.4%) versus 53 of 111 (47.7%), P = 0.002], as well as higher clinical benefit rate [84 of 98 (85.7%) versus 80 of 111 (72.1%), P = 0.017]. Multivariable logistic regression identified 5-FU use as the only independent predictor of response (OR 2.28, 95% confidence interval 1.23-4.23, P = 0.009). Median overall survival was longer in the 5-FU group compared with non-5-FU (21.0 versus 14.0 months, P = 0.008), as was median PFS1 (15.2 versus 6.3 months, P < 0.001). Multivariable Cox regression confirmed 5-FU therapy as an independent prognostic factor for longer PFS1 (hazard ratio 0.50, 95% confidence interval 0.31-0.80, P = 0.004).
Conclusions: CDX2-positive CUP represents a distinct subgroup with favorable response to 5-FU-based therapy, supporting CDX2 as a predictive biomarker for treatment selection.
Keywords: 5-fluorouracil; biomarker; cancer of unknown primary; caudal type homeobox 2.
Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure The authors have declared no conflicts of interest.
Figures
Similar articles
-
Chemotherapy for advanced gastric cancer.Cochrane Database Syst Rev. 2017 Aug 29;8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4. Cochrane Database Syst Rev. 2017. PMID: 28850174 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Zanidatamab plus chemotherapy as first-line treatment for patients with HER2-positive advanced gastro-oesophageal adenocarcinoma: primary results of a multicentre, single-arm, phase 2 study.Lancet Oncol. 2025 Jul;26(7):847-859. doi: 10.1016/S1470-2045(25)00287-6. Epub 2025 Jun 2. Lancet Oncol. 2025. PMID: 40473445 Clinical Trial.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.Health Technol Assess. 2008 May;12(15):iii-ix, xi-162. doi: 10.3310/hta12150. Health Technol Assess. 2008. PMID: 18462574
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
References
-
- Greco F.A. Cancer of unknown primary site: still an entity, a biological mystery and a metastatic model. Nat Rev Cancer. 2014;14(1):3–4. - PubMed
-
- Rassy E., Pavlidis N. The currently declining incidence of cancer of unknown primary. Cancer Epidemiol. 2019;61:139–141. - PubMed
-
- Hemminki K., Bevier M., Hemminki A., Sundquist J. Survival in cancer of unknown primary site: population-based analysis by site and histology. Ann Oncol. 2012;23(7):1854–1863. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

