Endothelial-Mesenchymal Transition in Tumor Microenvironment Promotes Neuroendocrine Differentiation of Prostate Cancer
- PMID: 40706636
- PMCID: PMC12485659
- DOI: 10.1111/cas.70144
Endothelial-Mesenchymal Transition in Tumor Microenvironment Promotes Neuroendocrine Differentiation of Prostate Cancer
Abstract
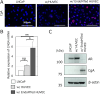
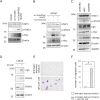
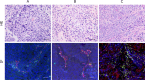
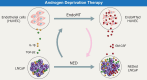
Neuroendocrine prostate cancer (NEPC) is a highly aggressive and treatment-resistant subtype of castration-resistant prostate cancer (CRPC) that often emerges during progression under androgen-receptor (AR) pathway inhibition. While lineage plasticity in cancer cells has been recognized as a key mechanism of resistance, the role of the tumor microenvironment in driving this transition remains unclear. Among its cellular components, vascular endothelial cells can undergo endothelial-mesenchymal transition (EndoMT), a phenotypic shift associated with tumor progression and fibrosis. Here, we investigated whether EndoMT contributes to NEPC development. Human umbilical vein endothelial cells (HUVEC) were induced to undergo EndoMT using IL-1β and TGF-β2, and are hereafter referred to as EndoMTed HUVEC. EndoMTed HUVEC promoted neuroendocrine features and functional changes in LNCaP cells. Transcriptome analysis revealed marked upregulation of granulocyte-macrophage colony-stimulating factor (GM-CSF) in EndoMTed HUVEC. Neutralization of GM-CSF signaling using mavrilimumab, a monoclonal antibody targeting the GM-CSF receptor alpha (CSF2RA), and siRNA-mediated CSF2RA knockdown both suppressed the neuroendocrine phenotype and STAT3 signaling of LNCaP cells. Conversely, GM-CSF stimulation alone reproduced these changes. Enzalutamide-treated LNCaP cells secreted IL-1β and TGF-β2, which in turn triggered EndoMT, suggesting a reciprocal loop. These findings indicate that anti-androgen therapy may inadvertently promote NEPC through a paracrine loop involving tumor-derived cytokines and endothelial GM-CSF secretion, highlighting EndoMT as a microenvironmental driver of treatment resistance.
Keywords: androgen deprivation therapy; endothelial‐mesenchymal transition; granulocyte‐macrophage colony‐stimulating factor; neuroendocrine differentiation; prostate cancer.
© 2025 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Registry and the Registration No. of the Study/Trial: Not applicable.
Animal Studies: All animal experiments were approved by the Animal Research Committee of Mie University (approval number: 2019–32).
The authors declare no conflicts of interest.
Figures



References
-
- Maman S. and Witz I. P., “A History of Exploring Cancer in Context,” Nature Reviews Cancer 18, no. 6 (2018): 359–376. - PubMed
-
- Zeisberg E. M., Potenta S., Xie L., Zeisberg M., and Kalluri R., “Discovery of Endothelial to Mesenchymal Transition as a Source for Carcinoma‐Associated Fibroblasts,” Cancer Research 67, no. 21 (2007): 10123–10128. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

