Combined dietary omega-3 and omega-6 fatty acids protect against hyperglycemia-associated retinopathy in neonatal mice
- PMID: 40706777
- PMCID: PMC12445345
- DOI: 10.1016/j.phrs.2025.107877
Combined dietary omega-3 and omega-6 fatty acids protect against hyperglycemia-associated retinopathy in neonatal mice
Abstract
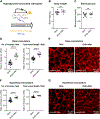
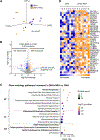
Retinopathy of prematurity (ROP) with early vessel loss (Phase I) followed by uncontrolled vessel growth (Phase II) causes visual impairment in premature infants. Although supplementation with omega-3 (n-3) docosahexaenoic acid (DHA) alone shows mixed results in preventing ROP, supplementation with both n-3 DHA and n-6 arachidonic acid (ARA) in early postnatal life reduces severe ROP by 50 % (Mega Donna Mega study). In the Mega Donna Mega study, 146 (72.6 %) of 201 included infants had at least one hyperglycemic episode during the first 14 days of life, which is a strong ROP risk factor. We therefore evaluated the protective effects and mechanisms of combined dietary n-3 DHA and n-6 ARA in a neonatal mouse model of hyperglycemia-induced suppression of retinal vascular development (Phase I ROP). At postnatal day (P) 10, retinal vessel growth was improved in pups from mothers on diets enriched with 1 % DHA + 2 % ARA vs. 3 % DHA. Lipid changes in pup plasma and RPE complex (retinal pigment epithelium with choroid and sclera) were in accordance with maternal diets' DHA and ARA levels, indicating that milk lipids reflected maternal diets. Proteomic retinal analysis revealed increased abundances of proteins related to mitochondrial respiration and glucose metabolism with the combined diet. Inhibition of mitochondrial ATP synthase negated the protective effects of the combined diet. In conclusion, combined DHA+ARA oral maternal supplementation protects against hyperglycemia-induced retinopathy in mouse neonates (Phase I ROP model) through enhanced retinal metabolism, suggesting the potential of balanced lipid supplementation for ROP prevention.
Keywords: ARA; DHA; Postnatal hyperglycemia; Retinal vasculature; Retinopathy of prematurity.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have declared no conflict of interest.
Figures


References
-
- Garcia H, Villasis-Keever MA, Zavala-Vargas G, Bravo-Ortiz JC, Perez-Mendez A, Escamilla-Nunez A, Global prevalence and severity of retinopathy of prematurity over the last four decades (1985–2021): a systematic review and meta-analysis, Arch. Med Res 55 (2) (2024) 102967. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

