Development of Broad-Spectrum Antimicrobial Peptides through the Conjugation of FtsZ-Binding and Cell-Penetrating Peptides
- PMID: 40707007
- PMCID: PMC12340945
- DOI: 10.1021/acsinfecdis.5c00220
Development of Broad-Spectrum Antimicrobial Peptides through the Conjugation of FtsZ-Binding and Cell-Penetrating Peptides
Abstract
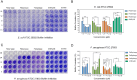
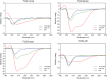
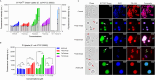
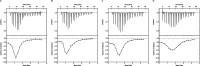
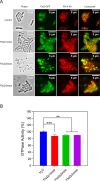
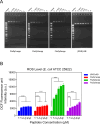
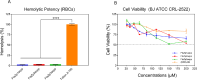
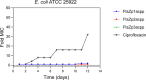
The rapid increase in bacterial resistance to conventional antibiotics has led to a great demand for novel antibacterial agents. Antimicrobial peptides (AMPs) are emerging as next-generation antimicrobial alternative drugs to conventional antibiotics because of their broad-spectrum antimicrobial activities and minimal potential for drug resistance induction. This work describes novel antimicrobial peptides (FtsZpcpp) synthesized through the conjugation of a cell penetration peptide ((RXR)4XB) to nonantimicrobial peptides (FtsZp). The FtsZp peptides were previously identified to bind FtsZ (flaming-temperature-sensitive protein Z), a crucial protein in regulating bacterial cell divisions. Newly designed FtsZpcpp peptides have broad antimicrobial activities against both Gram-positive and Gram-negative bacteria, including multidrug-resistant strains. Besides, these new peptides exert minimal hemolytic activity toward human red blood cells and low cytotoxicity toward human skin cells. Comprehensive studies on the antimicrobial mechanism of FtsZpcpp peptides revealed that they exert antimicrobial activities through multiple mechanisms, including membrane disruption and intracellular actions (e.g., interference with cell divisions, DNA binding, and reactive oxygen species (ROS) generation). Our results have shown that FtsZpcpp peptides have the potential to serve as future antimicrobial drugs in combating the increasing global problem of antibiotic resistance.
Keywords: antibiofilm; antimicrobial peptides; antimicrobial resistance; cell division; cell membrane; cell penetrating peptide.
Figures

References
-
- O’Neil, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations the Review on Antimicrobial Resistance. https://amr-review.org/Publications.html (accessed Apr 5, 2021).
-
- WHO. Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, 2024. License: CC BY-NC-SA 3.0 IGO.
-
- Boucher H. W., Talbot G. H., Benjamin D. K. J., Bradley J., Guidos R. J., Jones R. N., Murray B. E., Bonomo R. A., Gilbert D.. 10 x ’20 Progress-Development of New Drugs Active against Gram-Negative Bacilli: An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2013;56:1685–1694. doi: 10.1093/cid/cit152. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

