Constitutive IL-7 signaling promotes CAR-NK cell survival in the solid tumor microenvironment but impairs tumor control
- PMID: 40707132
- PMCID: PMC12315002
- DOI: 10.1136/jitc-2024-010672
Constitutive IL-7 signaling promotes CAR-NK cell survival in the solid tumor microenvironment but impairs tumor control
Abstract
Background: Adoptive transfer of chimeric antigen receptor (CAR)-expressing natural killer (NK) cells has demonstrated success against hematological malignancies. Efficacy against solid tumors has been limited by poor NK cell survival and function in the suppressive tumor microenvironment (TME). To enhance efficacy against solid tumors, stimulatory cytokines have been incorporated into CAR-NK cell therapeutic approaches. However, current cytokine strategies have limitations, including systemic toxicities, exogenous dependencies, and unwanted TME bystander effects. Here, we aimed to overcome these limitations by modifying CAR-NK cells to express a constitutively active interleukin (IL)-7 receptor, termed C7R, capable of providing intrinsic CAR-NK cell activation that does not rely on or produce exogenous signals nor activate bystander cells.
Methods: We examined persistence, antitumor function, and transcriptional profiles of CAR-NK cells coexpressing C7R in a novel tumor immune microenvironment (TiME) co-culture system and against hematologic and solid tumor xenografts in vivo.
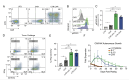
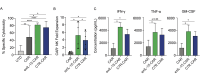
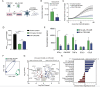
Results: Peripheral blood NK cells expressing a CAR directed against the solid tumor antigen GD2 and modified with C7R demonstrated enhanced tumor killing and persistence in vitro compared with CAR-NK cells without cytokine support and similar functions to CAR-NK cells supplemented with recombinant IL-15. C7R.CAR-NK cells exhibited enhanced survival and proliferation within neuroblastoma TiME xenografts in vivo but produced poor long-term tumor control compared with CAR-NK cells supplemented with IL-15. Similar results were seen using C7R-expressing CD19.CAR-NK cells against CD19+leukemia xenografts. Gene expression analysis revealed that chronic signaling via C7R induced a transcriptional signature consistent with intratumor stressed NK cells with blunted effector function. We identified gene candidates associated with chronic cytokine-stressed NK cells that could be targeted to reduce CAR-NK cell stress within the solid TME.
Conclusion: C7R promoted CAR-NK cell survival in hostile TMEs independent of exogenous signals but resulted in poor antitumor function in vivo. Our data reveals the detrimental role of continuous IL-7 signaling in CAR-NK cells and provides insights into proper application of cytokine signals when attempting to enhance CAR-NK cell antitumor activity.
Keywords: Adoptive cell therapy - ACT; Chimeric antigen receptor - CAR; Cytokine; Natural killer - NK; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: MD, IN, DvL, JP, CB, and RP declare no potential conflicts of interest. CMR declares FCOIs with Abintus (income), Allogene (stock), Bellicum Pharmaceuticals (IP ownership), Bluebird BIO INC (advisory board), Marker Therapeutics, LLC (equity), Memgen, LLC (income), Tessa Therapeutics PTE Ltd (income, equity), Tscan (income), Turnstone Biologics Ltd (income), and Walking Fish, LLC (stock). An FCOI Management Plan designed to safeguard objectivity and promote transparency in the research project ensuring, to the fullest extent possible, that the design, conduct, and reporting of this research is free from bias is in place at Baylor College of Medicine.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
