Self-driving microscopy detects the onset of protein aggregation and enables intelligent Brillouin imaging
- PMID: 40707429
- PMCID: PMC12289965
- DOI: 10.1038/s41467-025-60912-0
Self-driving microscopy detects the onset of protein aggregation and enables intelligent Brillouin imaging
Abstract
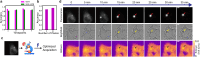
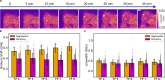
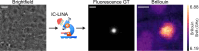
The process of protein aggregation, central to neurodegenerative diseases like Huntington's, is challenging to study due to its unpredictable nature and relatively rapid kinetics. Understanding its biomechanics is crucial for unraveling its role in disease progression and cellular toxicity. Brillouin microscopy offers unique advantages for studying biomechanical properties, yet is limited by slow imaging speed, complicating its use for rapid and dynamic processes like protein aggregation. To overcome these limitations, we developed a self-driving microscope that uses deep learning to predict the onset of aggregation from a single fluorescence image of soluble protein, achieving 91% accuracy. The system triggers optimized multimodal imaging when aggregation is imminent, enabling intelligent Brillouin microscopy of this dynamic biomechanical process. Furthermore, we demonstrate that by detecting mature aggregates in real time using brightfield images and a neural network, Brillouin microscopy can be used to study their biomechanical properties without the need for fluorescence labeling, minimizing phototoxicity and preserving sample health. This autonomous microscopy approach advances the study of aggregation kinetics and biomechanics in living cells, offering a powerful tool for investigating the role of protein misfolding and aggregation in neurodegeneration.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: H.A.L. has received funding from the industry to support research on neurodegenerative diseases, including from Merck Serono, UCB and AbbVie. These companies had no specific role in the conceptualization, preparation, or decision to publish this work. H.A.L. is also the co-founder and Chief Scientific Officer of ND BioSciences SA, a company that develops diagnostics and treatments for neurodegenerative diseases based on platforms that reproduce the complexity and diversity of proteins implicated in neurodegenerative diseases and their pathologies. All remaining authors declare no competing interests.
Figures


References
-
- Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci.4, 49–60 (2003). - PubMed
-
- Ross, C. A. & Poirier, M. A. Protein aggregation and neurodegenerative disease. Nat. Med.10, 10–17 (2004). - PubMed
-
- Walker, F. O. Huntington’s disease. Lancet369, 218–228 (2007). - PubMed
-
- Bates, G. P. et al. Huntington disease. Nat. Rev. Dis. Primers1, 1–21 (2015). - PubMed
-
- Davies, S. W. et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell90, 537–548 (1997). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

