Effect of naltrexone pretreatment on ketamine-induced glutamatergic activity and symptoms of depression: a randomized crossover study
- PMID: 40707608
- PMCID: PMC12443602
- DOI: 10.1038/s41591-025-03800-w
Effect of naltrexone pretreatment on ketamine-induced glutamatergic activity and symptoms of depression: a randomized crossover study
Abstract
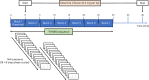
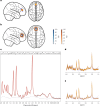
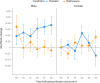
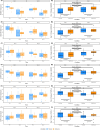
We investigated the potential role of the opioid system in modulating glutamatergic effects of ketamine administration in major depressive disorder. Twenty-six adults with major depressive disorder participated in a double-blind crossover study, receiving oral placebo or 50 mg naltrexone before an intravenous infusion of 0.5 mg per kg ketamine. Brain glutamatergic activity in the anterior cingulate cortex was measured using functional magnetic resonance spectroscopy and depressive symptoms were assessed with the Montgomery-Åsberg Depression Rating Scale. Naltrexone attenuated the increase in glutamate + glutamine to total N-acetylaspartate ratio during ketamine infusion compared to placebo (F1,253 = 4.83, P = 0.029) and also attenuated the reduction in Montgomery-Åsberg Depression Rating Scale scores on day 1 (condition-by-time interaction, F1,74 = 5.39, P = 0.023). These findings demonstrate that the opioid system modulates the acute response to ketamine and subsequent antidepressant effects. Interactions between the glutamate and opioid systems may have implications for the development of new depression treatment strategies. ClinicalTrials.gov registration: NCT04977674 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.H.Y. is employed by Imperial College London, is an Honorary Consultant at South London and Maudsley NHS Foundation Trust (NHS UK), editor of the Journal of Psychopharmacology and deputy editor of BJPsych Open. A.H.Y. has been paid for lectures and is on advisory boards for the following companies with drugs used in affective and related disorders: Flow Neuroscience, Novartis, Roche, Janssen, Takeda, Noema pharma, Compass, AstraZenaca, Boehringer Ingelheim, Eli Lilly, LivaNova, Lundbeck, Sunovion, Servier, Livanova, Janssen, Allegan, Bionomics, Sumitomo Dainippon Pharma, Sage and Neurocentrx. A.H.Y. is a principal investigator in the following: Restore-Life VNS registry study funded by LivaNova, ESKETINTRD3004: ‘An open-label, long-term, safety and efficacy study of intranasal esketamine in treatment-resistant depression’, ‘The effects of psilocybin on cognitive function in healthy participants’, ‘The safety and efficacy of psilocybin in participants with treatment-resistant depression (P-TRD)’, ‘A double-blind, randomized, parallel-group study with quetiapine extended release as comparator to evaluate the efficacy and safety of seltorexant 20 mg as adjunctive therapy to antidepressants in adult and elderly patients with major depressive disorder with insomnia symptoms who have responded inadequately to antidepressant therapy’ (Janssen), ‘An open-label, long-term, safety and efficacy study of aticaprant as adjunctive therapy in adult and elderly participants with major depressive disorder (MDD)’ (Janssen), ‘A randomized, double-blind, multicenter, parallel-group, placebo-controlled study to evaluate the efficacy, safety, and tolerability of aticaprant 10 mg as adjunctive therapy in adult participants with major depressive disorder (MDD) with moderate-to-severe anhedonia and inadequate response to current antidepressant therapy’, ‘A study of disease characteristics and real-life standard of care effectiveness in patients with major depressive disorder (MDD) with anhedonia and inadequate response to current antidepressant therapy including an SSRI or SNR’ (Janssen) as well as UK Chief Investigator for Compass (COMP006 & COMP007 studies) and Novartis MDD study (MIJ821A12201). A.H.Y. has received grant funding (past and present) from: NIMH (USA), CIHR (Canada), NARSAD (USA), Stanley Medical Research Institute (USA), MRC (UK), Wellcome Trust (UK), Royal College of Physicians (Edinburgh, UK), BMA (UK), UBC-VGH Foundation (Canada), WEDC (Canada), CCS Depression Research Fund (Canada), MSFHR (Canada), NIHR (UK) and Janssen (UK) EU Horizon 2020. M.A.M. has current funding from Nxera, Takeda and Lundbeck and has acted as an advisor for Boehringer Ingelheim, Neurocentrx, Quolet Pharmaceuticals and Nxera. The remaining authors declare no competing interests.
Figures


References
-
- Jelen L. A. & Stone J. M. Ketamine for depression. Int. Rev. Psychiatry33, 207–228 (2021). - PubMed
-
- Marcantoni, W. S. et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009–January 2019. J. Affect Disord.277, 831–841 (2020). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

