Quantifying clinical and genetic factors influencing rate and severity of autosomal dominant tubulointerstitial kidney disease progression
- PMID: 40707830
- PMCID: PMC12289749
- DOI: 10.1007/s10928-025-09989-0
Quantifying clinical and genetic factors influencing rate and severity of autosomal dominant tubulointerstitial kidney disease progression
Abstract
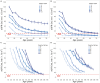
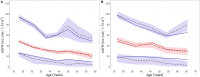
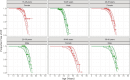
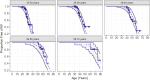
Autosomal dominant tubulointerstitial kidney disease (ADTKD), caused by mutations in UMOD and MUC1 genes, leads to tubular damage and fibrosis, ultimately resulting in kidney failure (KF). This study investigated clinical and genetic factors influencing the rate and severity of ADTKD progression by developing quantitative models. An estimated glomerular filtration rate (eGFR) of 10 mL/min/1.73 m2 was used to define KF, corresponding to dialysis initiation. Natural history data from the Wake Forest University School of Medicine study were used to develop the models for UMOD (n = 371) and MUC1 (n = 233) disease types (age ≥ 18 years). Longitudinal change in eGFR and time-to-KF were quantified using nonlinear mixed-effects and parametric time-to-event modeling approaches, respectively, in Monolix (version 2024R1). Sigmoid Imax functions with steepness parameters varying before and after inflection points best captured eGFR decline. Patients with UMOD and MUC1 disease variants exhibited a similar initial shallow steepness ( 1), but after inflection, each declined rapidly. MUC1 patients progressed faster than UMOD during the post-inflection phase (γ₂ = 10.23 vs. 6.34). eGFR at first clinic visit (eGFR_FCV) and age at first clinic visit (AFCV) significantly affected between-subject variability in eGFR decline. A Weibull hazard function best described the time to KF. In UMOD, males reached Te (the age at which approximately 36.8% of individuals remain free from KF) 4 years earlier than females on average (β_Te_Male = -0.07), indicating faster progression in males. Older AFCV was associated with slower progression to KF (β_Te_AFCV = 0.59 for UMOD and 0.81 for MUC1). These models may help enable quantitative data-driven subgroup analysis in the future, optimizing inclusion/exclusion criteria for ADTKD clinical trials.
Keywords: Baseline characteristics; Disease progression model; Rare kidney disease; eGFR.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures
References
-
- Devuyst O, Olinger E, Weber S, Eckardt KU, Kmoch S, Rampoldi L et al (2019) Autosomal dominant tubulointerstitial kidney disease. Nat Rev Dis Primers 5(1):60 - PubMed
-
- Eckardt KU, Alper SL, Antignac C, Bleyer AJ, Chauveau D, Dahan K et al (2015) Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management—A KDIGO consensus report. Kidney Int 88(4):676–683 - PubMed
-
- Olinger E, Hofmann P, Kidd K, Dufour I, Belge H, Schaeffer C et al (2020) Clinical and genetic spectra of autosomal dominant tubulointerstitial kidney disease due to mutations in UMOD and MUC1. Kidney Int 98(3):717–731 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

