EphrinB2 alleviates tubulointerstitial fibrosis in diabetic kidney disease
- PMID: 40707969
- PMCID: PMC12291390
- DOI: 10.1186/s12967-025-06852-1
EphrinB2 alleviates tubulointerstitial fibrosis in diabetic kidney disease
Abstract
Background: Diabetic kidney disease (DKD) is characterized by progressive fibrosis, oxidative stress, and mitochondrial dysfunction, contributing to renal dysfunction. EphrinB2, a cell surface protein, has been implicated in tissue repair and fibrosis, but its role in DKD remains poorly understood. This study investigates the impact of EphrinB2 expression on renal fibrosis, mitochondrial dynamics, and cellular signaling pathways in DKD.
Methods: EphrinB2 expression and function were investigated in renal tissues from DKD patients, STZ-induced diabetic mice, and HG-treated HK-2 cells. EphrinB2 overexpression was achieved using AAV in vivo and lentiviral vectors in vitro. Functional assessments included histological and biochemical evaluations, while mechanistic studies utilized siRNA knockdown, pathway-specific inhibitors and activators, and co-immunoprecipitation to explore the role of the Epac1-Rap1 signaling pathway in EphrinB2-mediated antifibrotic and mitochondrial protective effects.
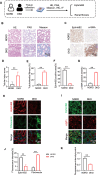
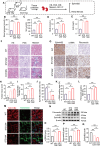
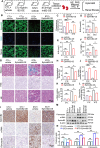
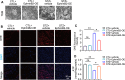
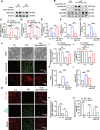
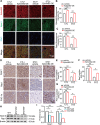
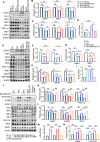
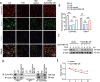
Results: EphrinB2 expression was significantly downregulated in the kidneys of DKD patients and STZ-induced diabetic mice, correlating with increased fibrosis and tubular injury. Overexpression of EphrinB2 (EphrinB2-OE) in diabetic mice restored renal function, reduced fibrosis, alleviated oxidative stress, and preserved mitochondrial structure. In HK-2 cells, EphrinB2-OE mitigated HG-induced fibrosis, reduced ROS levels, and restored MMP and ATP production. Mechanistically, EphrinB2-OE enhanced the Epac1-Rap1 pathway, stabilizing Epac1 protein and promoting mitochondrial biogenesis via PGC-1α. Additionally, EphrinB2-OE modulated the E-cadherin/β-catenin complex and preventing β-catenin nuclear translocation, and preserving epithelial integrity and epithelial-to-mesenchymal transition (EMT).
Conclusions: EphrinB2 exerts protective effects against renal fibrosis and dysfunction in diabetic conditions by regulating fibrosis pathways, mitochondrial dynamics, and epithelial stability. Targeting EphrinB2 signaling presents a promising therapeutic strategy for diabetic kidney disease.
Keywords: Diabetic kidney disease (DKD); Epac1-Rap1 signaling; EphrinB2; Mitochondrial dynamics; Oxidative stress; Renal fibrosis; β-catenin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experimental protocols were approved by the Clinical Trial Ethical Committee [2023255 K] and Animal Care and Ethics Committee of Wuhan University [ZN2023225]. Consent for publication: All authors have approved the final manuscript for publication. Competing interests: The authors declare that they have no competing interests.
Figures


References
-
- Lu Q, Yang L, Xiao JJ, Liu Q, Ni L, Hu JW, et al. Empagliflozin attenuates the renal tubular ferroptosis in diabetic kidney disease through AMPK/NRF2 pathway. Free Radic Biol Med. 2023;195:89–102. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

