Hypoxia-augmented chemotherapy potentiates imaging-guided combinatorial radionuclide-sonodynamic therapy for pancreatic cancer
- PMID: 40707984
- PMCID: PMC12288203
- DOI: 10.1186/s12951-025-03611-8
Hypoxia-augmented chemotherapy potentiates imaging-guided combinatorial radionuclide-sonodynamic therapy for pancreatic cancer
Abstract
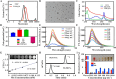
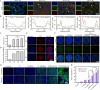
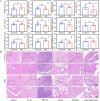
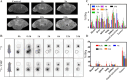
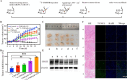
Radionuclide therapy and chemotherapy are effective for pancreatic cancer, yet their efficacy is often limited by tumor hypoxia. In this study, manganese porphyrin (MnTTP) and tirapazamine (TPZ) were encapsulated in polylactic-co-glycolic acid (PLGA) spheres, which were subsequently coated with polydopamine to label the radionuclide 131I, forming a theranostic nanoplatform. The nanoplatform demonstrated excellent biocompatibility, stable labeling efficiency, and dual-modal MRI/SPECT imaging capabilities. The nanoplatform generated reactive oxygen species (ROS) under ultrasound(US) activation, in combination with the β-rays emitted by 131I, synergistically eradicate tumor cells and exacerbate hypoxia in the tumor microenvironment. Furthermore, TPZ was activated to produce toxic free radicals under hypoxic conditions, enabling a synergistic therapeutic approach that combined radionuclide therapy and sonodynamic therapy. This approach effectively inhibited tumor stem cell formation and enhanced anti-tumor efficacy. Additionally, the nanoplatform's metabolism in vivo and the therapeutic effect were monitored in real-time under MRI/SPECT dual-modality imaging. This therapeutic strategy offers a promising solution for overcoming tumor hypoxia and achieving efficient combination therapy for tumors.
Keywords: Hypoxia-activated chemotherapy; Radionuclide therapy; SPECT imaging; Sonodynamic therapy; Synergistic therapeutic.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Tang Q, Wang Y, Wu R, et al. Spatial-temporal-controlled NO gas and PARP1 SiRNA delivery for alleviating the dilemma of deeper sonodynamic therapy in pancreatic cancer. Chem Eng J. 2024;490(15):151775. 10.1016/j.cej.2024.151775. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

