Harnessing Nanobodies for Precision Targeting of Proteoforms: Opportunities and Challenges in Therapeutics and Diagnostics
- PMID: 40708125
- PMCID: PMC12362336
- DOI: 10.1021/acschembio.5c00329
Harnessing Nanobodies for Precision Targeting of Proteoforms: Opportunities and Challenges in Therapeutics and Diagnostics
Abstract
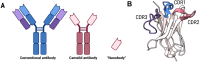
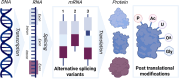
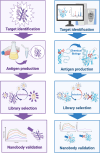
Proteoforms are biologically distinct yet structurally similar proteins that play key roles in driving disease progression but are rarely accounted for in the development of therapeutics and diagnostics. Nanobodies (Nbs) have emerged as a therapeutic and diagnostic "silver bullet" as they possess unique structural and functional attributes that offer advantages over traditional antibodies. One of the most profound advantages of Nbs is the heightened sensitivity and ability to distinguish subtle changes in the conformation of a given protein. Thus, Nbs have significant potential as therapeutic and diagnostic agents that can identify and distinguish specific pathological proteoforms that underlie a given disease. However, there remain significant challenges in obtaining sufficient quantities and purities of specific proteoform antigens that are required for engineering proteoform-specific Nbs. Recent advancements in chemical biology tools for precision proteoform synthesis have made this task feasible for the first time. In this perspective, we discuss the advantages and challenges associated with developing proteoform-specific Nbs and how success in this endeavor will significantly advance the fields of therapeutics and diagnostics.
Figures
References
-
- Zottel A., Jovcevska I., Samec N., Mlakar J., Sribar J., Krizaj I., Skoblar Vidmar M., Komel R.. Anti-vimentin, anti-TUFM, anti-NAP1L1 and anti-DPYSL2 nanobodies display cytotoxic effect and reduce glioblastoma cell migration. Ther. Adv. Med. Oncol. 2020;12:1758835920915302. doi: 10.1177/1758835920915302. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

