Sophoridine inhibits proliferation and migration by targeting PIM1 in breast cancer
- PMID: 40708207
- PMCID: PMC12302398
- DOI: 10.1080/13880209.2025.2537123
Sophoridine inhibits proliferation and migration by targeting PIM1 in breast cancer
Abstract
Context: Sophoridine, an alkaloid quinolizidine derived from Sophora flavescens Aiton (Fabaceae), has strong anti-tumor activity in a variety of malignancies. Nevertheless, the effects and underlying mechanism of sophoridine on breast cancer are not fully understood.
Objective: To identify the key targets and potential pharmacological mechanisms of sophoridine against breast cancer.
Materials and methods: MCF-10A, MCF-7 and MDA-MB-231 cells were treated with sophoridine for 24 or 48 h. MTT, colony formation assay, flow cytometry, wound healing, and Transwell assay were employed to illustrate the anti-tumor effects of sophoridine on breast cancer. Network pharmacology and molecular docking were used to determine the targets for sophoridine in breast cancer, and confirmed by molecular dynamics simulation and CETSA-western blot assay. Additionally, the functional rescue and signaling pathway regulated by sophoridine was analyzed.
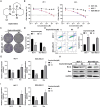
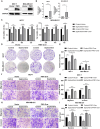
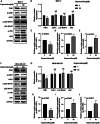
Results: Sophoridine suppressed the proliferation, migration, and invasion of breast cancer cells. The IC50 value of sophoridine for 48 h in MCF-10A, MCF-7 and MDA-MB-231 was 363 μM, 87.96 μM and 81.07 μM, respectively. PIM1 was the key target for sophoridine in breast cancer. Furthermore, PIM1 overexpression significantly reversed the suppressive impacts of sophoridine on growth and migration in breast cancer cells. Mechanistically, sophoridine inhibited the phosphorylation of ASK1 and activated JNK/p38 MAPK signaling pathway by downregulating PIM1 expression, and thus exhibited anti-tumor effects.
Discussion and conclusion: Taken together, sophoridine relies on targeting PIM1 to inhibit cell proliferation and migration in breast cancer, which might be related to the activation of ASK1/MAPK axis, suggesting the therapeutic potential of sophoridine for breast cancer.
Keywords: ASK1/MAPK pathway; Breast cancer; PIM1; Sophoridine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
Umbilical cord-derived mesenchymal stem cells promote proliferation and migration in MCF-7 and MDA-MB-231 breast cancer cells through activation of the ERK pathway.Oncol Rep. 2015 Sep;34(3):1469-77. doi: 10.3892/or.2015.4109. Epub 2015 Jul 3. Oncol Rep. 2015. PMID: 26151310
-
Discovery of a novel hybrid coumarin-hydroxamate conjugate targeting the HDAC1-Sp1-FOSL2 signaling axis for breast cancer therapy.Cell Commun Signal. 2024 Jul 15;22(1):361. doi: 10.1186/s12964-024-01733-4. Cell Commun Signal. 2024. PMID: 39010083 Free PMC article.
-
Pro-Apoptotic Activity of 1-(4,5,6,7-Tetrabromo-1H-benzimidazol-1-yl)propan-2-one, an Intracellular Inhibitor of PIM-1 Kinase in Acute Lymphoblastic Leukemia and Breast Cancer Cells.Int J Mol Sci. 2025 Jun 19;26(12):5897. doi: 10.3390/ijms26125897. Int J Mol Sci. 2025. PMID: 40565356 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Brasó-Maristany F, Filosto S, Catchpole S, Marlow R, Quist J, Francesch-Domenech E, Plumb DA, Zakka L, Gazinska P, Liccardi G, et al. 2016. PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Nat Med. 22(11):1303–1313. doi: 10.1038/nm.4198. - DOI - PMC - PubMed
-
- Burley SK, Bhikadiya C, Bi C, Bittrich S, Chao H, Chen L, Craig PA, Crichlow GV, Dalenberg K, Duarte JM, et al. 2023. RCSB Protein Data Bank (RCSB.org): delivery of experimentally-determined PDB structures alongside one million computed structure models of proteins from artificial intelligence/machine learning. Nucleic Acids Res. 51(D1):D488–d508. doi: 10.1093/nar/gkac1077. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
