Disease Progression in Children With Friedreich Ataxia: Functional Performance and Other Outcome Assessments in the FACHILD Study
- PMID: 40708339
- PMCID: PMC12313166
- DOI: 10.1177/08830738251353475
Disease Progression in Children With Friedreich Ataxia: Functional Performance and Other Outcome Assessments in the FACHILD Study
Abstract
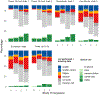
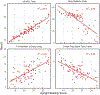
BackgroundFriedreich ataxia is a rare genetic disorder caused by mutations in the FXN gene, typically presenting with balance and coordination difficulties between ages 7 and 15 years. Neurologic symptoms are progressive and lead to loss of ambulation and especially in children other symptoms such as cardiomyopathy, scoliosis, and fatigue are common. The FACHILD natural history study aimed to expand knowledge about the disease course and evaluate clinical outcome assessments in children. We report on functional performance testing, clinical rating scales, and patient-reported outcomes as clinical outcome assessments for Friedreich ataxia. Over a 3-year period, all tests and assessments were conducted to evaluate their sensitivity to progression and correlate with established measures such as neurologic rating scales.MethodsIndividuals with genetically confirmed Friedreich ataxia, aged 7-18 years, were enrolled from October 2017 to November 2022. This analysis focused on ambulatory individuals, including timed walks (25-foot, 1 minute, and 6 minutes), the timed up and go, and the 9-hole pegboard test. Additionally, the Berg Balance Scale and FA-Activities of Daily Living were assessed. Progression data were analyzed using mixed models for repeated measures, with detailed analyses of intermittent missing data. Data from the Friedreich Ataxia Clinical Outcome Measures Study was used to augment analyses when available.Findings and InterpretationFunctional performance outcome measures are sensitive and clinically relevant tools for assessing disease progression in children with Friedreich ataxia. In early to moderately affected populations, the 1-Minute Walk demonstrated promising properties, showing comparable sensitivity to the modified Friedreich Ataxia Rating Scale and the Upright Stability Score.
Keywords: ataxia; outcome; pediatric.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CR reports consultancy fees from FARA, the National Ataxia Foundation, Biogen, PTC, Biohaven, Santhera, and Solaxa; DRL receives (outside the present study) grants from the NIH, FARA, MDA, Design, Retrotope, PTC, Reata, Stealth, Voyager, and Novartis. The remaining authors report no competing interests.
Figures



References
-
- Rummey C; Flynn JM; Corben LA; Delatycki MB; Wilmot G; Subramony SH; Bushara K; Duquette A; Gomez CM; Hoyle JC; Roxburgh R; Seeberger L; Yoon G; Mathews KD; Zesiewicz T; Perlman S; Lynch DR Scoliosis in Friedreich’s Ataxia: Longitudinal Characterization in a Large Heterogeneous Cohort. Annals of Clinical and Translational Neurology 2021, 8 (6), 1239–1250. 10.1002/acn3.51352. - DOI - PMC - PubMed
-
- Lynch DR; Hauser L; McCormick A; Wells M; Dong YN; McCormack S; Schadt K; Perlman S; Subramony SH; Mathews KD; Brocht A; Ball J; Perdok R; Grahn A; Vescio T; Sherman JW; Farmer JM Randomized, Double-Blind, Placebo-Controlled Study of Interferon- γ 1b in Friedreich Ataxia. Annals of Clinical and Translational Neurology 2019, 6 (3), 546–553. 10.1002/acn3.731. - DOI - PMC - PubMed
-
- PTC Therapeutics Announces Topline Results from Vatiquinone MOVE-FA Registration-Directed Trial ∣ PTC Therapeutics, Inc. https://ir.ptcbio.com/news-releases/news-release-details/ptc-therapeutic... (accessed 2023-07-20).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

