CRISPR/Cas9 editing of CBP80 enhances drought tolerance in potato (Solanum tuberosum)
- PMID: 40708586
- PMCID: PMC12287025
- DOI: 10.3389/fpls.2025.1598947
CRISPR/Cas9 editing of CBP80 enhances drought tolerance in potato (Solanum tuberosum)
Erratum in
-
Correction: CRISPR/Cas9 editing of CBP80 enhances drought tolerance in potato (Solanum tuberosum).Front Plant Sci. 2025 Aug 22;16:1674362. doi: 10.3389/fpls.2025.1674362. eCollection 2025. Front Plant Sci. 2025. PMID: 40918959 Free PMC article.
Abstract
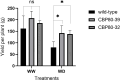
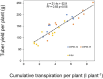
Developing drought-tolerant potato varieties is increasingly important due to climate change and water scarcity, as potatoes are highly sensitive to water deficits that can significantly reduce yield and tuber quality. The cap-binding protein CBP80, involved in the abscisic acid (ABA) signalling pathway, has emerged as a promising target for improving drought tolerance in plants. In this study, we used CRISPR/Cas9 to edit the StCBP80 gene in the tetraploid potato cultivar Spunta. Given the complexity of editing all four alleles in a tetraploid genome, eight independent partially edited lines (two or three alleles edited) were obtained. Two of these lines were selected for detailed molecular and phenotypic characterization. Under restricted water conditions, the selected lines exhibited reduced transpiration rates and improved leaf area index compared to non-edited controls. Gene expression analysis by quantitative real-time PCR showed differential expression of drought-responsive genes (P5CS, PDH, and MYB33), supporting a role for StCBP80 in stress response modulation. Moreover, the edited lines showed lower yield penalties, both in biomass and tuber production, under drought stress. This work represents one of the first applications of genome editing to enhance drought tolerance in a commercial potato cultivar, and highlights CBP80 as a promising target for crop improvement. These findings provide valuable insights for the development of stress-resilient potato varieties using genome editing approaches.
Keywords: abiotic stress; abscisic acid; cap binding proteins; climate change; genome edited plants.
Copyright © 2025 Decima Oneto, Massa, Echarte, Rey Burusco, Gonzalez, Alfonso, Laserna, Norero, Divito and Feingold.
Conflict of interest statement
The reviewer EA declared a shared affiliation with the authors MG and PL to the handling editor at the time of review.
Figures








References
-
- Alves A., Cordeiro D., Correia S., Miguel C. (2021). Small non-coding RNAs at the crossroads of regulatory pathways controlling somatic embryogenesis in seed plants. Plants 10 (3), 504. Available at: https://www.mdpi.com/2223-7747/10/3/504., PMID: - PMC - PubMed
-
- Andersson M., Turesson H., Nicolia A., Fält A.-S., Samuelsson M., Hofvander P. (2018). Efficient targeted multiallelic mutagenesis in tetraploid potato (Solanum tuberosum) by transient CRISPR-Cas9 expression in protoplasts. Plant Cell Rep. 37, 1–13. doi: 10.1111/ppl.12731, PMID: - DOI - PMC - PubMed
-
- Andrade Díaz D. (2024). Association Study of SNPs Markers to Traits Linked to Drought Stress Tolerance in Potato [Art. 277]. Int. J. Life Sci. Agric. Res. doi: 10.55677/ijlsar/V03I5Y2024-08 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

