Testosterone differentially modulates the display of agonistic behavior and dominance over opponents before and after adolescence in male Syrian hamsters
- PMID: 40708844
- PMCID: PMC12287037
- DOI: 10.3389/fnbeh.2025.1603862
Testosterone differentially modulates the display of agonistic behavior and dominance over opponents before and after adolescence in male Syrian hamsters
Abstract
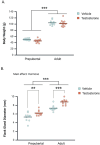
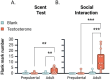
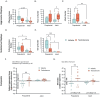
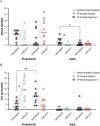
The current study investigated the influence of testosterone on agonistic behavior and dominance over an opponent before and after adolescence in male Syrian hamsters (Mesocricetus auratus), and tested the hypothesis that shifts in behavioral responsiveness to testosterone occur across adolescent development. We predicted that testosterone-dependent modulation of attacks decreases following puberty, and that flank marking behavior in response to testosterone increases following puberty. Prepubertal (14 days of age) and adult subjects (52-62 days of age) were gonadectomized and immediately implanted with testosterone propionate (TP) or vehicle pellets. Fourteen days later, agonistic behaviors were assessed in a neutral arena with age-matched testosterone-treated opponents. TP treatment increased attacks and dominance over an opponent in prepubertal but not adult males, supporting the hypothesis that testosterone-dependent modulation of aggression decreases following puberty. TP increased flank marking behavior in adults, but failed to increase flank marking in prepubertal subjects, supporting the hypothesized increase in testosterone-dependent modulation of flank marking after puberty. Thus, we provide here evidence that changes in agonistic responses to steroid hormones occur across puberty and adolescence in male rodents, much like the well-established shifts in neuroendocrine and reproductive behavioral responses to steroid hormones that occur pre- to post-pubertally. These findings may have implications for early pubertal timing and increased risk for externalizing symptoms and aggressive behavior in humans.
Keywords: adolescence; aggressive; agonistic; dominance; puberty; social behavior; submissive; testosterone.
Copyright © 2025 Castaneda, Whitten, Menard, Sisk, Cooper and Schulz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Update of
-
Testosterone differentially modulates the display of agonistic behavior and dominance over opponents before and after adolescence in male Syrian hamsters.bioRxiv [Preprint]. 2025 Apr 1:2025.03.31.646499. doi: 10.1101/2025.03.31.646499. bioRxiv. 2025. Update in: Front Behav Neurosci. 2025 Jul 10;19:1603862. doi: 10.3389/fnbeh.2025.1603862. PMID: 40236030 Free PMC article. Updated. Preprint.
References
-
- Albers H. E., Huhman K. L., Meisel R. L. (2002). Hormonal basis of social conflict and communication. Horm. Brain Behav. 1 393–433. 10.1016/B978-012532104-4/50008-1 - DOI
-
- Bartter F. C., Sniffen R. C., Simmons F. A., Albright F., Howard R. P. (1952). Effects of chorionic gonadotropin (APL) in male “eunuchoidism with low folliclestimulating hormone”: Aqueous solution versus oil and beeswax suspension*†. J. Clin. Endocrinol. Metab. 12 1532–1550. 10.1210/jcem-12-12-1532 - DOI - PubMed
LinkOut - more resources
Full Text Sources

