A rapid-release pure iodine coating on titanium implants to mitigate acute periprosthetic infections
- PMID: 40708857
- PMCID: PMC12287049
- DOI: 10.3389/fbioe.2025.1590411
A rapid-release pure iodine coating on titanium implants to mitigate acute periprosthetic infections
Abstract
Background: Periprosthetic infections remain a significant challenge in orthopedic surgeries, primarily due to bacterial biofilm formation on implant surfaces. To address this issue, we developed a novel iodine-based coating on titanium implants designed to rapidly release iodine, thereby preventing acute infections. The efficacy and safety of this coating were assessed through both in vitro experiments and an in vivo rabbit model.
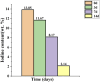
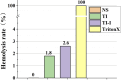
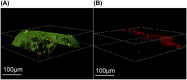
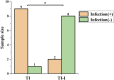
Methods: The iodine coating was applied to titanium implants using electrophoretic deposition. The coated implants were characterized using scanning electron microscopy (SEM), X-ray fluorescence spectroscopy (XRF), and energy-dispersive spectroscopy (EDS). In vitro studies included antibacterial assays, iodine release kinetics, and hemolysis tests. Additionally, an acute periprosthetic infection model in rabbits was established to evaluate the coating's performance in vivo.
Results: The electrophoretic deposition technique successfully produced a uniform iodine coating with high iodine content and rapid release kinetics. In vitro tests demonstrated significant antibacterial activity against Staphylococcus aureus and Escherichia coli. The rabbit model showed a marked reduction in infection rates compared to uncoated implants, with no adverse effects on bone integration.
Conclusion: This study introduces a promising iodine-based coating for titanium implants, offering a rapid and effective solution to prevent acute periprosthetic infections while maintaining biocompatibility and supporting bone healing.
Keywords: biofilm prevention; infections; iodine; orthopedic implants; titanium.
Copyright © 2025 Zhang, Liang, Chen, Huang, Wang, Chen and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Akkaya M., Zanna L., Sangaletti R., Bokhari A., Gehrke T., Citak M. (2023). What is the Most reliable concordance rate of preoperative synovial fluid aspiration and intraoperative biopsy to detect periprosthetic joint infection in knee, hip and shoulder arthroplasty? Antibiot. (Basel) 12, 1482. 10.3390/antibiotics12101482 - DOI - PMC - PubMed
-
- Coppola G. A., Onsea J., Moriarty T. F., Nehrbass D., Constant C., Zeiter S., et al. (2021). An improved 2-aminoimidazole based anti-biofilm coating for orthopedic implants: activity, stability, and in vivo biocompatibility. Front. Microbiol. 12, 658521. 10.3389/fmicb.2021.658521 - DOI - PMC - PubMed
-
- Dale H., Høvding P., Tveit S. M., Graff J. B., Lutro O., Schrama J. C., et al. (2021). Increasing but levelling out risk of revision due to infection after total hip arthroplasty: a study on 108,854 primary THAs in the Norwegian arthroplasty register from 2005 to 2019. Acta Orthop. 92, 208–214. 10.1080/17453674.2020.1851533 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

