Multiaxial rotational loading compromises the transition zone of the intervertebral disc: Ex vivo study using next-generation bioreactors
- PMID: 40708985
- PMCID: PMC12284434
- DOI: 10.1002/btm2.70033
Multiaxial rotational loading compromises the transition zone of the intervertebral disc: Ex vivo study using next-generation bioreactors
Abstract
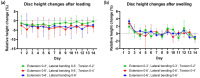
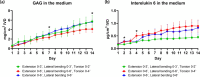
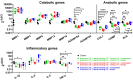
Bioreactors have become indispensable tools in spine research, enabling long-term intervertebral disc culture under controlled biological and mechanical conditions. Conventional systems are often limited to uniaxial loading, restricting their ability to replicate the complex, multidirectional biomechanics of the spine. To overcome this limitation, we developed a next-generation bioreactor capable of simulating multiaxial motions while preserving the disc's biological environment. In this study, we investigated the effects of complex loading patterns on early disc degeneration by subjecting bovine whole-organ discs to combined extension, lateral bending, and torsion at 0.3 Hz for 2 h daily over 14 days. To assess the impact of loading magnitude and the specific contribution of torsion, discs were exposed to either low- or high-angle rotations, with or without torsional loading at higher angles. Histological analysis revealed a marked loss of glycosaminoglycans (GAG) and collagen type II within the inner annulus fibrosus and transitional nucleus pulposus (NP), encompassing the transition zone (TZ), as well as GAG depletion in the central NP. Matrix degradation was observed across all loading conditions, with the most severe breakdown occurring under high-angle extension, bending, and torsion. All loading regimes induced cell death in the TZ and central NP, although torsion-free loading better maintained cell viability. These findings highlight the TZ, alongside the commonly affected NP, as a critical early site of degeneration. The study further underscores the importance of incorporating multiaxial loading in disc degeneration models and provides new insights into the biomechanical mechanisms underlying disc pathology.
Keywords: bioreactors; intervertebral disc; multiaxial loading; transition zone; whole organ culture.
© 2025 The Author(s). Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
All authors declare that they have no conflicts of interest with respect to this work.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

