Preventing opioid prescribing for low back pain using multimodal mechanical stimulation vs. TENS: a randomized-controlled trial
- PMID: 40709052
- PMCID: PMC12287057
- DOI: 10.3389/fpain.2025.1612572
Preventing opioid prescribing for low back pain using multimodal mechanical stimulation vs. TENS: a randomized-controlled trial
Abstract
Background: Low back pain (LBP) is the most common reason for outpatient opioid prescribing: a quarter of patients receive prescriptions, leading to opioid use disorder (OUD) in 5%. Guideline-recommended multimodal interventions often face implementation barriers, and effective modalities (e.g., electrical stimulation) lack coverage. A multimodal mechanical stimulation (M-Stim) device for LBP has demonstrated safety and efficacy in pain reduction, but its impact on opioid use has not yet been determined.
Methods: As part of an NIH-funded double-blind study to reduce pain and opioid use, patients with moderate-to-severe LBP presenting to two suburban chiropractic centers were randomized to receive either the M-Stim device or a transcutaneous electrical nerve stimulation (TENS) unit for 30 min daily, in addition to other therapies. Analgesic use was reported daily for 28 days, with new prescribing followed weekly for 3 months. The primary outcome was prescribing in the opioid-naïve subjects. Secondary endpoints included risk factors for prolonged use in the opioid-naïve subjects, milligram morphine equivalents (MME) for opioid users between the first and last 2 weeks, and prescribing compared with national rates.
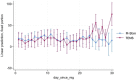
Results: After informed consent, 159 eligible patients were randomized to M-Stim (87) or TENS (72) (mean age 42.6 years, 54% female, BMI 30.9, NRS 5.5) between 23 June 2022 and 31 December 2023. Zero opioid-naïve M-Stim participants (n = 43) received prescriptions (0% vs. 8.6%, Fisher's exact p = 0.086), and those taking opioids used significantly fewer MME [7.5 (SD 3.54) vs. 498.5 MME (SD 474.9), p < 0.0001] for fewer of reported days [M-Stim 2/47 (4.2%)] compared with TENS [n = 36, 38/102 (37%), RR 0.11 (95% CI 0.28-0.44), p = 0.0018]. M-Stim significantly reduced MME in opioid users [-44.6% (32.33 MME), p = 0.02], use days for those with BMI ≥30 [-3 (99% CI -5.73 to -0.26), p = 0.032], and prescribing compared with national rates [9.8% vs. 25%, -63%, RR 0.32 (95% CI 0.16-0.66), p = 0.002] while TENS did not.
Conclusions: Among chiropractic patients with moderate-to-severe LBP, added use of a multimodal M-Stim device in the opioid-naïve subjects significantly reduced factors associated with OUD compared with TENS and reduced use days for those with BMI ≥30. This novel device is a potential alternative to prescribing opioids as first line for LBP management.
Clinical trial registration: https://clinicaltrials.gov/study/NCT04491175, identifier NCT04491175.
Keywords: DuoTherm; M-Stim; TENS; focal vibration; low back pain; multifidus; opioid.
© 2025 Baxter, Etnoyer-Slaski, Williams, Swartout, Cohen and Lawson.
Conflict of interest statement
AB is employed by Harmonic Scientific LLC. AB reports grants from the National Institutes of Health during the conduct of the study and is the inventor and owner of the DuoTherm technology. She receives no corporate fees or royalties but is on the scientific advisory board of Bright Therapeutics. During the time conducting the study, she received honoraria or speaking travel stipends from the Society for Pediatric Sedation, Voices for Non-opioid Choices, PROSA European Pediatric Sedation and Anesthesia Conference, the European Pediatric Anesthesia Society, and the Australia Child Life Association. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Delitto A, Patterson CG, Stevans JM, Brennan GP, Wegener ST, Morrisette DC, et al. PCORI Final Research Reports. Comparing Ways to Treat Low Back Pain and Prevent Chronic Pain and Disability—the TARGET Trial. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI) (2021). Copyright © 2021. University of Pittsburgh. All Rights Reserved. - PubMed
-
- Deyo RA, Hallvik SE, Hildebran C, Marino M, Dexter E, Irvine JM, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naïve patients: a statewide retrospective cohort study. J Gen Intern Med. (2017) 32(1):21–7. 10.1007/s11606-016-3810-3 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

