Adverse effects of antiseizure medications: a review of the impact of pharmacogenetics and drugs interactions in clinical practice
- PMID: 40709084
- PMCID: PMC12287013
- DOI: 10.3389/fphar.2025.1584566
Adverse effects of antiseizure medications: a review of the impact of pharmacogenetics and drugs interactions in clinical practice
Abstract
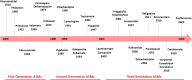
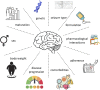
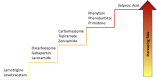
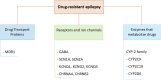
Epilepsy is a chronic and debilitating neurological disorder characterized by the occurrence of spontaneous and recurrent seizures. Despite the availability of several antiseizure medications (ASMs), people with epilepsy often experience drug resistance and adverse effects. This narrative review provides an overview of the main adverse drug reactions (ADR) caused by ASMs, including neurological, metabolic, skin reactions and drug failure, and of the underlying molecular mechanisms. Given the critical contribution of pharmacogenomics and drug-drug interactions to the occurrence of some ADRs, we provide examples of the role of major allelic variations identified in genes encoding for molecules involved in the pharmacokinetics, pharmacodynamics and immune system and emphasize the activity of ASMs as inhibitors or inducers of metabolic enzymes. Improved knowledge of the benefit-risk profile of drugs, also through enhanced pharmacovigilance activity and following guidelines recommendations, could implement patients care avoiding ADRs and favoring a beneficial personalized medicine particularly in vulnerable patients as children, elderly people and pregnant women.
Keywords: adverse drug reactions; antiseizure medications; drug interactions; epilepsy; pharmacogenetics.
Copyright © 2025 De Bellis, d’Orsi, Rubino, Arigliano, Carella, Sciruicchio, Liantonio, De Luca and Imbrici.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Alvarado A. T., Muñoz A. M., Varela N., Sullón-Dextre S., Pineda M., Bolarte-Arteaga M., et al. (2023). Pharmacogenetic variants of CYP2C9 and CYP2C19 associated with adverse reactions induced by antiepileptic drugs used in Peru. Pharm. Rev. 70 (3), 603–618. 10.3897/pharmacia.70.e109011 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials