A diaryl urea derivative, SMCl inhibits cell proliferation through the RAS/RAF/MEK/ERK pathway in hepatocellular carcinoma
- PMID: 40709085
- PMCID: PMC12287612
- DOI: 10.3389/fphar.2025.1605515
A diaryl urea derivative, SMCl inhibits cell proliferation through the RAS/RAF/MEK/ERK pathway in hepatocellular carcinoma
Abstract
Introduction: Hepatocellular carcinoma (HCC) ranks among the three most prevalent cancer-related diseases in terms of incidence. Hence, exploring drugs for HCC therapy is of great significance. Compounds with a diaryl urea structure have been reported to exhibit a broad range of biological activities, including anticancer activity. This study focuses on the specific diaryl urea derivative 4-(4-(3-(2-chloro-3-(trifluoromethyl)phenyl)ureido)phenoxy)-N-methylpicolinamide (SMCl), with particular emphasis on investigating its therapeutic effects against hepatocellular carcinoma (HCC) and elucidating the underlying molecular mechanisms.
Methods: In vitro anti-cancer effects of SMCl were evaluated in HCC cell lines using MTS, colony formation, and wound healing assays. Western blot analyzed RAS/RAF/MEK/ERK pathway modulation. In vivo efficacy was assessed using a xenograft model.
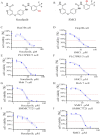
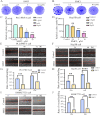
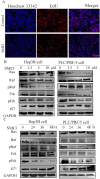
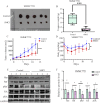
Results: The MTS and colony formation assays demonstrated that SMCl significantly decreased the viability of HCC cells. Western blot analysis demonstrated that SMCl effectively suppressed hepatocellular carcinoma proliferation by markedly inhibiting the RAS/RAF/MEK/ERK signaling pathway, with this inhibitory effect exhibiting both time- and concentration-dependent characteristics. SMCl also demonstrated significant therapeutic efficacy in the xenograft tumor model, achieving a tumor inhibition rate of 72.37%. Notably, it showed no significant impact on spleen weight or body weight in mice, indicating low toxicity to normal tissues.
Conclusion: This study first elucidates the effects of SMCl on HCC cells and its impact on the RAS/RAF/MEK/ERK signaling pathway, providing a potential active compound for the clinical treatment of liver cancer.
Keywords: RAS/RAF/MEK/ERK; bioactive compound; cell proliferation; diaryl urea derivative; hepatocellular carcinoma.
Copyright © 2025 Fu, Fang, Qiu, Lai, Xu, Chen, Li and Zhu.
Conflict of interest statement
Authors FQ, JL, YX, and BC were employed by Shenzhen ChemStrong Scientific Co., Ltd. Author YL was employed by Shenzhen Jiangchuan Pharmaceutical Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Ciprofol exerts anti-tumour effects in hepatocellular carcinoma through the Raf-MEK-ERK signalling pathway.Transl Gastroenterol Hepatol. 2025 May 13;10:52. doi: 10.21037/tgh-24-115. eCollection 2025. Transl Gastroenterol Hepatol. 2025. PMID: 40755729 Free PMC article.
-
Downregulation of Raf-1 kinase inhibitory protein as a sorafenib resistance mechanism in hepatocellular carcinoma cell lines.J Cancer Res Clin Oncol. 2018 Aug;144(8):1487-1501. doi: 10.1007/s00432-018-2672-y. Epub 2018 Jun 1. J Cancer Res Clin Oncol. 2018. PMID: 29858683 Free PMC article.
-
Non-canonical activation of MAPK signaling by the lncRNA ASH1L-AS1-encoded microprotein APPLE through inhibition of PP1/PP2A-mediated ERK1/2 dephosphorylation in hepatocellular carcinoma.J Exp Clin Cancer Res. 2025 Jul 11;44(1):200. doi: 10.1186/s13046-025-03465-w. J Exp Clin Cancer Res. 2025. PMID: 40646641 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

