Identified Medications Causing Medication-Related Osteonecrosis of the Jaw: A Literature Review
- PMID: 40709114
- PMCID: PMC12287985
- DOI: 10.7759/cureus.86645
Identified Medications Causing Medication-Related Osteonecrosis of the Jaw: A Literature Review
Abstract
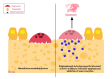
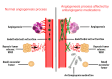
Medication-related osteonecrosis of the jaw (MRONJ) is a serious adverse event that occurs in patients treated with certain medications, leading to a major side effect that can result in substantial morbidity. MRONJ is known to be associated with antiresorptive (AR) and antiangiogenic (AA) medicines, but recent evidence suggests that other medications may also be involved. Recognizing a broad range of drug-induced risk factors is important, notably in light of the increasing number of drugs associated with MRONJ. The primary objective of this review is to update and synthesize findings from the past 22 years (2003 to April 2025) regarding medications associated with MRONJ, expanding beyond the traditional AR and AA drugs to include more recent and developing therapeutic classes. This review combines findings from recent literature, including studies on AR and AA medications, as well as other drug classes, such as targeted cancer therapies and immunomodulatory agents. This review provides updated insights into MRONJ for healthcare practitioners and dentists, emphasizing the significance of risk assessment, early recognition, and multidisciplinary management to improve patient care and outcomes. Given its potential to cause significant morbidity and complicate dental and medical treatments, MRONJ presents a critical concern in clinical practice, underscoring the need for heightened awareness among healthcare professionals.
Keywords: antiangiogenic drugs; antiresorptive agents; drug-induced osteonecrosis; medication-related osteonecrosis of the jaw (mronj); mronj prevention.
Copyright © 2025, Baghalipour et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures

Similar articles
-
Interventions for managing medication-related osteonecrosis of the jaw.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD012432. doi: 10.1002/14651858.CD012432.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866376 Free PMC article.
-
Interventions for managing medication-related osteonecrosis of the jaw.Cochrane Database Syst Rev. 2017 Oct 6;10(10):CD012432. doi: 10.1002/14651858.CD012432.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Jul 12;7:CD012432. doi: 10.1002/14651858.CD012432.pub3. PMID: 28983908 Free PMC article. Updated.
-
The Use of Human Amniotic Membrane (hAM) as a Treatment Strategy of Medication-Related Osteonecrosis of the Jaw (MRONJ): A Systematic Review and Meta-Analysis of the Literature.Medicina (Kaunas). 2023 May 17;59(5):968. doi: 10.3390/medicina59050968. Medicina (Kaunas). 2023. PMID: 37241200 Free PMC article.
-
Awareness and Attitude Towards MRONJ Among Physicians Prescribing Antiresorptive Drugs: A Cross-Sectional Study.BMC Oral Health. 2025 Jul 2;25(1):985. doi: 10.1186/s12903-025-06490-5. BMC Oral Health. 2025. PMID: 40604825 Free PMC article.
-
How does medication-related osteonecrosis of the jaw (MRONJ) influence the health-related quality of life after surgery?BMC Oral Health. 2025 Jun 21;25(1):951. doi: 10.1186/s12903-025-06171-3. BMC Oral Health. 2025. PMID: 40544272 Free PMC article.
References
-
- American Association of Oral and Maxillofacial Surgeons' position paper on medication-related osteonecrosis of the jaws—2022 update. Ruggiero SL, Dodson TB, Aghaloo T, Carlson ER, Ward BB, Kademani D. J Oral Maxillofac Surg. 2022;80:920–943. - PubMed
-
- Medication-related osteonecrosis of the jaw: a narrative review of risk factors, diagnosis, and management. Thomas JG, Ouanounou A. Front Oral Maxillofac Med. 2023;5:31.
-
- Medication-related osteonecrosis of the jaw: analysing the range of implicated drugs from the Australian database of adverse event notifications. Teoh L, Moses G, Nguyen AP, McCullough MJ. Br J Clin Pharmacol. 2021;87:2767–2776. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
