Grass carp Trim47 restricts GCRV infection via SPRY domain-mediated autophagic degradation of nonstructural proteins and disruption of viral inclusion bodies
- PMID: 40709172
- PMCID: PMC12286827
- DOI: 10.3389/fimmu.2025.1623014
Grass carp Trim47 restricts GCRV infection via SPRY domain-mediated autophagic degradation of nonstructural proteins and disruption of viral inclusion bodies
Abstract
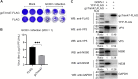
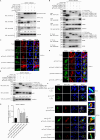
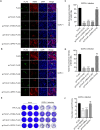
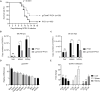
Trim47, a TRIM C-VII subgroup protein characterized by a conserved SPRY domain, has been primarily studied for its ubiquitin-dependent roles in mammals. This study reports a paradigm-shifting finding in teleost immunology: grass carp Trim47 (gcTrim47) employs its SPRY domain to execute a novel, ubiquitin-independent antiviral pathway, selectively degrading GCRV-I nonstructural proteins NS38/NS80 via autophagy-mediated clearance. Unlike mammalian TRIMs, gcTrim47 antiviral activity is strictly dependent on its SPRY domain-devoid of RING/B-box domains critical for E3 ligase function-revealing an evolutionarily divergent mechanism where substrate-targeting specificity, not ubiquitination, drives viral replication factory (viral inclusion body, VIB) dismantling. Functional assays demonstrated that gcTrim47 overexpression in CIK cells reduced viral titers and suppressed VIB formation, with SPRY domain deletion ablating these effects. In vivo, a yeast surface-display platform presenting gcTrim47-PYD1 conferred 32.94% relative percent survival (RPS) against GCRV-II infection, the first reported use of a TRIM family protein as an antiviral immunogen in grass carp. This strategy mitigated splenic/kidney viral loads and alleviated histopathological damage, including tubular necrosis and inflammatory infiltration. The successful application of this mechanism into a yeast-based immunization strategy highlights its potential for developing novel antiviral biotherapeutics in aquaculture.
Keywords: SPRY domain; autophagic degradation; gcTRIM47-PYD1 recombinant Saccharomyces cerevisiae biologics; grass carp Trim47; grass carp reovirus.
Copyright © 2025 Yan, Chen, Yan, Zhang and Chang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

