Gut-tropic α4β7+CD8+ T cells contribute to pancreatic β cell destruction in type 1 diabetes
- PMID: 40709181
- PMCID: PMC12286794
- DOI: 10.3389/fimmu.2025.1623428
Gut-tropic α4β7+CD8+ T cells contribute to pancreatic β cell destruction in type 1 diabetes
Abstract
Background: T cells are crucial in destroying pancreatic β cells, resulting in insulitis in type 1 diabetes (T1D). However, only 1% to 2% of infiltrating CD8+ T cells are specific for islet autoantigens. The mechanisms driving non-cognate T cells to the islets and their potential pathogenic roles remain unclear.
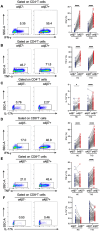
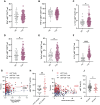
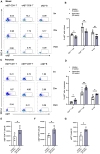
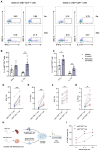
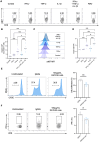
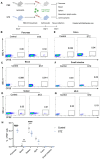
Methods: We analyzed the frequency and function of circulating gut-tropic immune cells in 99 patients with T1D and 57 healthy controls. We also analyzed single-cell RNA sequencing on pancreata from 10 T1D donors, 11 autoantibody-positive donors, and 15 non-diabetic controls. Correlation analysis was performed to elucidate the relationship between gut-tropic cells and clinical variables. In NOD mice, we examined gut-tropic T cell frequencies, cytokine profiles, and cytotoxicity at different disease stages. Additionally, we investigated the role of integrin α4β7 on gut-tropic T cells function and migration.
Results: Gut-tropic CD8+ T cells are reduced in peripheral blood but elevated in pancreatic islets of patients with T1D, correlating with impaired β-cell function. Gut-tropic CD8+ T cells exhibited stronger cytokine production than non-gut-tropic counterparts. In NOD mice, gut-tropic cells increased in the islets and decreased in the blood during insulitis progression. Gut-tropic CD8+ T cells showed augmented cytokine production and cytotoxicity against islet cells. Integrin α4β7 was a key mediator of the pathogenicity of CD8+ T cells and upregulated by the inflammatory signals. Insulitis directly drove gut-tropic CD8+ T cells migrating to inflamed islets.
Conclusions: Gut-tropic CD8+ T cells bridge the intestinal immune system and the pathogenesis of T1D, offering potential biomarkers and therapeutic targets.
Keywords: autoimmunity; gut tropic T cells; integrin α4β7; islet function; type 1 diabetes.
Copyright © 2025 Su, Bian, Zhao, Cai, Yang, Li and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The extra-islet pancreas supports autoimmunity in human type 1 diabetes.Elife. 2025 Apr 15;13:RP100535. doi: 10.7554/eLife.100535. Elife. 2025. PMID: 40232951 Free PMC article.
-
Beta cell extracellular vesicle PD-L1 as a novel regulator of CD8+ T cell activity and biomarker during the evolution of type 1 diabetes.Diabetologia. 2025 Feb;68(2):382-396. doi: 10.1007/s00125-024-06313-2. Epub 2024 Nov 7. Diabetologia. 2025. PMID: 39508879
-
Pancreatic beta cell autophagy is impaired in type 1 diabetes.Diabetologia. 2021 Apr;64(4):865-877. doi: 10.1007/s00125-021-05387-6. Epub 2021 Jan 30. Diabetologia. 2021. PMID: 33515072 Free PMC article.
-
The emerging relationship between mucosal-associated invariant T cell populations and the onset and progression of type 1 diabetes.Front Immunol. 2025 Jun 18;16:1602934. doi: 10.3389/fimmu.2025.1602934. eCollection 2025. Front Immunol. 2025. PMID: 40607421 Free PMC article. Review.
-
Means, Motive, and Opportunity: Do Non-Islet-Reactive Infiltrating T Cells Contribute to Autoimmunity in Type 1 Diabetes?Front Immunol. 2021 Jun 16;12:683091. doi: 10.3389/fimmu.2021.683091. eCollection 2021. Front Immunol. 2021. PMID: 34220832 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

