Single-cycle, pseudotyped reporter influenza virus to facilitate evaluation of treatment strategies for avian influenza, Ebola and other highly infectious diseases in vivo
- PMID: 40709188
- PMCID: PMC12286815
- DOI: 10.3389/fimmu.2025.1608074
Single-cycle, pseudotyped reporter influenza virus to facilitate evaluation of treatment strategies for avian influenza, Ebola and other highly infectious diseases in vivo
Abstract
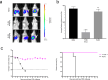
The rapid spread of infectious diseases presents a significant global threat, with seasonal influenza viruses, leading to 290,000-650,000 deaths annually. Emerging high pathogenic influenza strains from animals such as H5N1 and H7N9 further exacerbates pandemic risks. While developing effective vaccines and therapeutics is critical, the evaluation of these interventions is constrained by the requirement for high biosafety containment facilities. To circumvent these challenges, we developed S-Lux, a replication-deficient, single-cycle recombinant influenza virus expressing firefly luciferase (Flux) as a reporter protein. S-Lux can be pseudotyped with haemagglutinin from avian influenza, H5 and H7, enabling real-time monitoring of viral infection in vivo, and facilitate therapeutic antibody evaluation in low-containment facilities. In mice, S-Lux infection resulted in dose-dependent bioluminescent expression in the mouse airways and allowed evaluation of neutralising monoclonal antibodies and clearance of infected cells in mice. To extend this system, we generated ES-Lux by pseudotyping with the Ebola Glycoprotein (GP) and demonstrated that ES-Lux can be used to evaluate the efficacy of Ebola GP-targeting antibodies in vivo. Together, S-Lux and ES-Lux enable robust, simple and time-efficient assessment of antiviral therapy targeting influenza and Ebola virus in vivo, overcoming biosafety constraints that limit traditional efficacy studies.
Keywords: bioluminescence; in vivo imaging; influenza; pandemic; reporter virus.
Copyright © 2025 Tan, Rijal, Huang, Hyde, Gill and Townsend.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- World Health Organisation . Influenza (Seasonal) Fact Sheets. WHO; (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal).
-
- Cowling BJ, Jin L, Lau EH, Liao Q, Wu P, Jiang H, et al. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet. (2013) 382:129–37. doi: 10.1016/S0140-6736(13)61171-X, PMID: - DOI - PMC - PubMed
-
- Wang X, Jiang H, Wu P, Uyeki TM, Feng L, Lai S, et al. Epidemiology of avian influenza A H7N9 virus in human beings across five epidemics in mainland China 2013-17: an epidemiological study of laboratory-confirmed case series. Lancet Infect Dis. (2017) 17:822–32. doi: 10.1016/S1473-3099(17)30323-7, PMID: - DOI - PMC - PubMed
-
- Centers For Disease Control and Prevention . Viruses of Special Concern (2023). Available online at: https://www.cdc.gov/flu/pandemic-resources/monitoring/viruses-concern.html (Accessed August 31, 2023).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

