Interactions between atrial fibrosis and inflammation in atrial fibrillation
- PMID: 40709209
- PMCID: PMC12287038
- DOI: 10.3389/fcvm.2025.1578148
Interactions between atrial fibrosis and inflammation in atrial fibrillation
Abstract
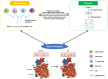
Atrial fibrillation (AF) is a complex arrhythmia driven by intricate pathophysiological mechanisms, with atrial fibrosis and inflammation emerging as central players in its initiation and perpetuation. Key pathways, including the renin-angiotensin-aldosterone system (RAAS), TGF-β/Smad signaling, and pro-inflammatory cytokine cascades (e.g., TNF-α/NF-κB, IL-6/STAT3), contribute to fibrotic remodeling and electrophysiological dysfunction. These pathways promote extracellular matrix deposition, fibroblast activation, and heterogeneous conduction, creating a substrate for AF maintenance. Contemporary therapeutic approaches predominantly target rhythm control via catheter ablation techniques and pharmacological interventions with antiarrhythmic agents. Nevertheless, the efficacy of anti-inflammatory approaches, such as corticosteroids and colchicine, remains uncertain due to limited robust clinical evidence, highlighting the need for further investigation. Advanced fibrosis quantification modalities, particularly late gadolinium-enhanced magnetic resonance imaging and electroanatomic mapping, have emerged as valuable tools for optimizing ablation strategies. Furthermore, emerging evidence highlights significant sex-based disparities in atrial fibrosis distribution and electrophysiological substrate characteristics, suggesting the potential for gender-specific therapeutic approaches. This comprehensive review systematically examines the pathophysiological roles of atrial fibrosis and inflammation in AF progression, with particular emphasis on their intricate bidirectional relationship. Through detailed elucidation of these mechanistic interactions, we aim to facilitate the development of novel therapeutic interventions to enhance clinical management of AF.
Keywords: atrial; atrial fibrillation; atrial fibrosis; fibrillation; fibrosis; inflammation.
© 2025 Pang, Ren and Yao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
TGF-β-Driven Atrial Fibrosis in Atrial Fibrillation: From Mechanistic Insights to Targeted Therapies.Aging Dis. 2025 Jul 31. doi: 10.14336/AD.2025.0564. Online ahead of print. Aging Dis. 2025. PMID: 40768638 Review.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.Health Technol Assess. 2008 Nov;12(34):iii-iv, xi-xiii, 1-198. doi: 10.3310/hta12340. Health Technol Assess. 2008. PMID: 19036232
-
External electrical and pharmacological cardioversion for atrial fibrillation, atrial flutter or atrial tachycardias: a network meta-analysis.Cochrane Database Syst Rev. 2024 Jun 3;6(6):CD013255. doi: 10.1002/14651858.CD013255.pub2. Cochrane Database Syst Rev. 2024. PMID: 38828867 Free PMC article.
-
Metabolic-dysfunction associated steatotic liver disease and atrial fibrillation: A review of pathogenesis.World J Cardiol. 2025 Jun 26;17(6):106147. doi: 10.4330/wjc.v17.i6.106147. World J Cardiol. 2025. PMID: 40575425 Free PMC article. Review.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

