Junctophilin-2 Regulates Store-Operated Calcium Entry to Drive Cardiac Fibroblast Activation, Fibrotic Repair, and Angiogenesis After Myocardial Infarction
- PMID: 40709455
- PMCID: PMC12456265
- DOI: 10.1161/CIRCULATIONAHA.125.073937
Junctophilin-2 Regulates Store-Operated Calcium Entry to Drive Cardiac Fibroblast Activation, Fibrotic Repair, and Angiogenesis After Myocardial Infarction
Erratum in
-
Correction to: Junctophilin-2 Regulates Store-Operated Calcium Entry to Drive Cardiac Fibroblast Activation, Fibrotic Repair, and Angiogenesis After Myocardial Infarction.Circulation. 2025 Nov 4;152(18):e371. doi: 10.1161/CIR.0000000000001398. Epub 2025 Nov 3. Circulation. 2025. PMID: 41183168 No abstract available.
Abstract
Background: Calcium (Ca2+) homeostasis in cardiac fibroblasts (CFs) plays a critical role in myocardial repair and remodeling after injury. JPH2 (junctophilin-2; human JPH2 or mouse Jph2) is a structural protein known to regulate intracellular Ca2+ signaling and excitation-contraction coupling in cardiomyocytes. However, the role of JPH2 in CF biology remains unexplored.
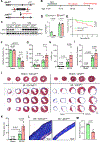
Methods: Junctophilin expression was assayed in human and mouse CFs using reverse transcription quantitative polymerase chain reaction, Western blotting, and immunofluorescence. To investigate the functional role of Jph2 in CFs, we assessed Ca2+ handling with live-cell confocal imaging, conducted RNA sequencing analysis, and assayed TGFβ (transforming growth factor β) responses after adenovirus-mediated gene silencing of Jph2 and fibroblast-specific Jph2 knockout. Jph2 interactions in CFs were identified using immunoprecipitation and proximity biotin ligation assays and confirmed by coimmunoprecipitation and rescue experiments. The in vivo role of Jph2 in CFs was investigated in fibroblast-specific Jph2 knockout mice (Col1a2CreERT/Jph2flox/flox, Jph2fKO) at baseline and after myocardial infarction and evaluated for changes in cardiac function, fibrosis, angiogenesis, CF activation, differentiation, and proliferation.
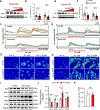
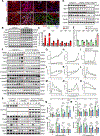
Results: Jph2 was identified as the only junctophilin expressed in CFs and acutely upregulated in response to myocardial infarction. Cellular and RNA sequencing analyses revealed that isolated Jph2-deficient CFs exhibited impaired fibroblast activation, reduced extracellular matrix production, and diminished expression of VEGFA (vascular endothelial growth factor A), VEGFB, and VEGFC after TGFβ treatment. In vivo, Jph2fKO mice displayed exacerbated adverse cardiac remodeling after myocardial infarction, characterized by decreased CF activation/extracellular matrix production, enhanced CF proliferation, worsened systolic dysfunction, increased left ventricular dilation, impaired scar maturation, and decreased angiogenesis. Mechanistically, biochemical assays demonstrated that Jph2 interacts directly with the coiled-coil 2 domain of Stim1 (stromal interaction molecule 1) via its joining region. Jph2 knockdown led to Stim1 protein destabilization, defective store-operated Ca2+entry, and a reduction in both canonical and noncanonical TGFβ signaling pathways. Stim1 overexpression partially rescued the sensitivity of Jph2-deficient CFs to TGFβ, as evidenced by increased expression of periostin and fibronectin-1, as well as enhanced Smad3 phosphorylation.
Conclusions: Our results demonstrate that Jph2 is required in CFs to orchestrate Ca2+ homeostasis, CF activation, and extracellular matrix production and promotes angiogenesis in the infarcted heart, positioning it as a central regulator of cardiac repair after injury.
Keywords: angiogenesis; extracellular matrix; fibroblasts; fibrosis; junctophilin-2; myocardial infarction; store-operated calcium entry.
Conflict of interest statement
Dr Song is an inventor on a patent regarding the use of junctophilin-2 fragments for the treatment of heart failure and other diseases (WO2017214296A1).
Figures
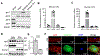



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

