Therapeutic plasma exchange in critically ill patients in low-income and lower-middle-income countries: medical need and feasibility
- PMID: 40709581
- PMCID: PMC12290984
- DOI: 10.7189/jogh.15.04214
Therapeutic plasma exchange in critically ill patients in low-income and lower-middle-income countries: medical need and feasibility
Abstract
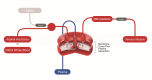
Background: Therapeutic plasma exchange (TPE) is a blood purification technique designed for the removal of large molecules such as pathogenic antibodies and lipoproteins. The procedure involves removing plasma from the patient in exchange for replacement fluid, and it can be performed either by membrane separation or centrifugation. These conventional techniques are expensive and require the training of skilled personnel. This severely limits their use in low-income countries (LICs) and lower-middle-income countries (LMICs), leading to morbidity and mortality for patients in LICs and LMICs suffering from the diseases where TPE is indicated.
Methods: A novel gravity-driven blood separation method might provide the needed access to TPE for LICs and LMICs. We reviewed the medical need, the practical aspects, as well as the possible complications of applying this novel technology in LICs and LMICs. Furthermore, we describe a feasibility study of implementing TPE in Suriname for various diseases and conditions.
Results: Where data was available (n /N = 10/11), supportive care combined with TPE using the new device resulted in improved values for the disease-specific markers evaluated in these patients. In addition, eight patients showed complete clinical recovery, and one patient showed partial clinical recovery upon TPE within 0.5-6 months of follow-up. Importantly, none of the patients experienced any serious side effects.
Conclusions: This experience in the resource-limited setting in Suriname illustrates that its application is feasible in LICs and LMICs settings, at least for these five diseases with first-line indications for TPE and a significant number of patients.
Copyright © 2025 by the Journal of Global Health. All rights reserved.
Conflict of interest statement
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and declare the following activities and/or relationships: APN is the inventor of the HemoClear filter, holds stock ownership in HemoClear BV, Ceintuurbaan 28, 8024 AA Zwolle, Netherlands. He is not involved in patient treatment in Suriname. Other authors disclose no relevant interest.
Figures


References
-
- Rososhansky S, Szymanski IO.Clinical Applications of Therapeutic Hemapheresis. J Intensive Care Med. 1996;11:149–61. 10.1177/088506669601100302 - DOI
-
- Connelly-Smith L, Alquist CR, Aqui NA, Hofmann JC, Klingel R, Onwuemene OA, et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice – Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Ninth Special Issue. J Clin Apher. 2023;38:77–278. 10.1002/jca.22043 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
