Off-pore Nucleoporin sPOM121 Transcriptionally Propels β-Catenin-driven Tumor Progression and Immune Escape in Prostate Cancer
- PMID: 40709833
- PMCID: PMC12580793
- DOI: 10.1158/2159-8290.CD-25-0629
Off-pore Nucleoporin sPOM121 Transcriptionally Propels β-Catenin-driven Tumor Progression and Immune Escape in Prostate Cancer
Abstract
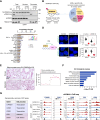
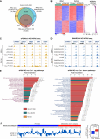
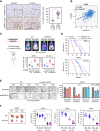
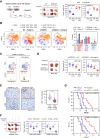
The roles of nucleoplasm-residing nucleoporins (NUP) in solid tumors, including prostate cancer, remain unknown. In this study, we reveal the clinical significance and mechanistic role of the off-pore NUP, soluble POM121 (sPOM121), as a crucial transcriptional regulator that enhances the aggressiveness of metastatic prostate cancer. Using orthogonal methodologies in human samples, sPOM121 was identified as the predominantly expressed nucleoplasmic NUP in prostate cancer. Unbiased proteomic and epigenomic studies demonstrate that sPOM121, through its C-terminus, interacts with the chromatin remodeler SMARCA5 at gene promoter sites and localizes at nuclear condensates, reprogramming gene expression. Indeed, sPOM121 regulates a distinct oncogenic gene network, including β-catenin, leading to prostate cancer progression and immune evasion. Importantly, targeting the sPOM121/β-catenin axis in patient-derived preclinical and syngeneic mouse models halts prostate cancer aggressiveness and enhances antitumor immunity. Taken together, these findings reveal previously unknown actionable reprogramming functions of off-pore NUPs in solid tumors.
Significance: This study uncovers how oncogenic signaling programs are transcriptionally heightened by the NUP sPOM121 in metastatic prostate cancer. Localization of sPOM121 at active transcriptional nuclear condensates propels disease progression and immune evasion, offering novel anticancer therapeutic opportunities.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
J.J. Orme reports grants from NIH and Prostate Cancer Foundation and nonfinancial support from Genentech outside the submitted work. S.M. Dehm reports personal fees from Oncternal Therapeutics, TCG Crossover, and American Association for Cancer Research and grants from NCI, US Department of Defense Prostate Cancer Research Program, and Prostate Cancer Foundation outside the submitted work. E.S. Antonarakis reports grants and personal fees from Janssen, Johnson & Johnson, Sanofi, Bayer, Bristol Myers Squibb, Convergent Therapeutics, Curium, MacroGenics, Merck, Pfizer, and AstraZeneca, personal fees from Aadi Bioscience, Abeona Therapeutics, Aikido Pharma, Astellas, Blue Earth, Boundless Bio, Corcept Therapeutics, Duality Bio, Exact Sciences, Hookipa Pharma, Invitae, Eli Lilly and Company, Foundation Medicine, Menarini-Silicon Biosystems, Tango Therapeutics, Tempus, Tolmar Scientific, VIR Biotechnology, and Z-alpha, and grants from Novartis, Celgene, and Orion outside the submitted work, as well as a patent for an AR-V7 biomarker technology that is issued and licensed to Qiagen. No disclosures were reported by the other authors.
Figures



References
MeSH terms
Substances
Grants and funding
- R01 CA294563/CA/NCI NIH HHS/United States
- Margaret Q. Landenberger Research Foundation (The Margaret Q. Landenberger Research Foundation)
- R01 CA285345/CA/NCI NIH HHS/United States
- R01CA294563/National Cancer Institute (NCI)
- R01 CA261925/CA/NCI NIH HHS/United States
- R01 CA286864/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- R01CA280999/National Cancer Institute (NCI)
- R01CA261925/National Cancer Institute (NCI)
- R01CA237398/National Cancer Institute (NCI)
- R01 CA237398/CA/NCI NIH HHS/United States
- R01 CA280999/CA/NCI NIH HHS/United States
- R01CA285345/National Cancer Institute (NCI)
- R01CA286864/National Cancer Institute (NCI)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

