Whole-Cell Fiber-Optic Biosensor for Real-Time, On-Site Sediment and Water Toxicity Assessment: Applications at Contaminated Sites Across Israel
- PMID: 40710054
- PMCID: PMC12293957
- DOI: 10.3390/bios15070404
Whole-Cell Fiber-Optic Biosensor for Real-Time, On-Site Sediment and Water Toxicity Assessment: Applications at Contaminated Sites Across Israel
Abstract
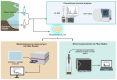
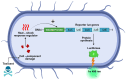
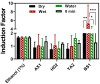
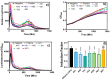
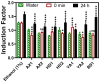
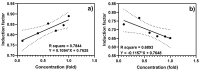
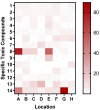
Sediments are key players in the optimum functioning of ecosystems; however, they also represent the largest known repository of harmful contaminants. The vast variety of these sediment-associated contaminants may exert harmful effects on marine communities and can impair ecosystem functioning. Whole-cell biosensors are a rapid and biologically relevant tool for assessing environmental toxicity. Therefore, in this study, we developed a bioassay-based toxicity measurement system using genetically modified bacteria to create a whole-cell optical biosensor. Briefly, reporter bacteria were integrated and immobilized using a calcium alginate matrix on fiber-optic tips connected to a photon counter placed inside a light-proof, portable case. The calcium alginate matrix acts as a semi-permeable membrane that protects the reporter-encapsulated optical fiber tips and allows the inward passage of toxicant(s) to induce a dose-dependent response in the bioreporter. The samples were tested by directly submerging the fiber tip with immobilized bacteria into vials containing either water or suspended sediment samples, and the subsequent bioluminescent responses were acquired. In addition to bioavailable sediment toxicity assessments, conventional chemical methods, such as liquid chromatography-mass spectroscopy (LC-MS) and inductively coupled plasma optical emission spectroscopy (ICP-OES), were used for comprehensive evaluation. The results demonstrated the efficacy of the biosensor in detecting various toxicity levels corresponding to identified contaminants, highlighting its potential integration into environmental monitoring frameworks for enhanced sediment and water quality assessments. Despite its utility, this study notes the system's operational challenges in field conditions, recommending future enhancements for improved portability and usability in remote locations.
Keywords: bioluminescent bacteria; bioreporter; in situ monitoring; optical-fiber biosensor; sediment toxicity; water toxicity; whole-cell biosensor.
Conflict of interest statement
R.S.M. is the co-founder of footprints. The other authors have no conflicts of interest to declare.
Figures





Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Electrophoresis.2025 Jul 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36251838 Free Books & Documents.
-
Optimized genetic circuitry and reporters for sensitive whole-cell arsenic biosensors: advancing environmental monitoring.Appl Environ Microbiol. 2025 Aug 20;91(8):e0060125. doi: 10.1128/aem.00601-25. Epub 2025 Jul 10. Appl Environ Microbiol. 2025. PMID: 40637449 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Massei R., Hollert H., Krauss M., Von Tümpling W., Weidauer C., Haglund P., Küster E., Gallampois C., Tysklind M., Brack W.J. Toxicity and neurotoxicity profiling of contaminated sediments from Gulf of Bothnia (Sweden): A multi-endpoint assay with Zebrafish embryos. Environ. Sci. Eur. 2019;31:8. doi: 10.1186/s12302-019-0188-y. - DOI
-
- Besten P.J.D., de Deckere E., Babut M.P., Power B., DelValls T.A., Zago C., Oen A.M.P., Heise S. Biological effects-based sediment quality in ecological risk assessment for European waters. J. Soils Sediments. 2003;3:144–162. doi: 10.1065/jss2003.08.084. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

