Recent Advances in Antibody Discovery Using Ultrahigh-Throughput Droplet Microfluidics: Challenges and Future Perspectives
- PMID: 40710059
- PMCID: PMC12293579
- DOI: 10.3390/bios15070409
Recent Advances in Antibody Discovery Using Ultrahigh-Throughput Droplet Microfluidics: Challenges and Future Perspectives
Abstract
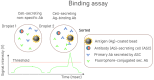
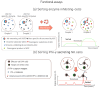
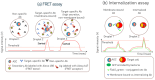
Droplet microfluidics has emerged as a transformative technology that can substantially increase the throughput of antibody "hit" discovery. This review provides a comprehensive overview of the recent advances in this dynamic field, focusing primarily on the technological and methodological innovations that have enhanced the antibody discovery process. This investigation starts with the fundamental principles of droplet microfluidics, emphasizing its unique capabilities for precisely controlling and manipulating picoliter-volume droplets. This discussion extends to various assay types employed in droplet microfluidics, including binding assays, functional assays, Förster Resonance Energy Transfer (FRET) assays, internalization assays, and neutralization assays, each offering distinct advantages for antibody screening and characterization. A critical examination of methods to improve droplet encapsulation is presented, besides addressing challenges such as reducing the leakage of small molecules from droplets and explaining what a "hit" droplet looks like. Furthermore, we assess design considerations essential for implementing high-throughput fluorescence-activated droplet sorting (FADS) workstations and emphasize the need for automation. This review also delves into the evolving commercial landscape, identifying key market players and emerging industry trends. This review paper aims to catalyze further research and innovation, ultimately advancing the field towards more efficient and robust solutions for antibody identification and development.
Keywords: FADS; FRET assay; antibody discovery; binding assay; droplet microfluidics; internalization assay; neutralization assay; sorting workstation; “hit” droplet.
Conflict of interest statement
All authors D.D., J.S.M., J.H.M., J.G. and D.B. are full-time employees of Ampersand Biomedicines, Inc., 135 William T. Morrissey Blvd., Boston, MA 02125, USA. Ampersand Biomedicines is a private biotechnology company focused on developing targeted therapeutics. For more information, please visit
Figures

Similar articles
-
Ultrahigh-throughput screening of environmental bacteria for proteolytic activity using droplet-based microfluidics.Appl Environ Microbiol. 2025 Jul 23;91(7):e0010925. doi: 10.1128/aem.00109-25. Epub 2025 Jun 13. Appl Environ Microbiol. 2025. PMID: 40511932 Free PMC article.
-
Microfluidics Based Particle and Droplet Generation for Gene and Drug Delivery Approaches.J Biomed Mater Res B Appl Biomater. 2025 Feb;113(2):e35530. doi: 10.1002/jbm.b.35530. J Biomed Mater Res B Appl Biomater. 2025. PMID: 39840932 Review.
-
Wood Waste Valorization and Classification Approaches: A systematic review.Open Res Eur. 2025 May 6;5:5. doi: 10.12688/openreseurope.18862.2. eCollection 2025. Open Res Eur. 2025. PMID: 40438563 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
High-throughput monoclonal antibody screening from immunized rabbits via droplet microfluidics.Lab Chip. 2025 Jul 8;25(14):3482-3494. doi: 10.1039/d5lc00340g. Lab Chip. 2025. PMID: 40468988
References
-
- Antibody Therapeutics Approved or in Regulatory Review in the EU or US. The Antibody Society. [(accessed on 12 January 2025)]. Available online: https://www.antibodysociety.org/resources/approved-antibodies/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

