Droplet-Based Measurements of DNA-Templated Nanoclusters-Towards Point-of-Care Applications
- PMID: 40710067
- PMCID: PMC12294068
- DOI: 10.3390/bios15070417
Droplet-Based Measurements of DNA-Templated Nanoclusters-Towards Point-of-Care Applications
Abstract
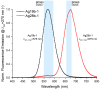
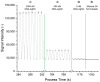
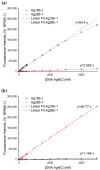
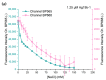
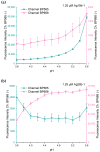
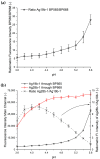
In this work, we investigate the fundamental usability of fluorescent DNA-templated silver nanoclusters (DNA-AgNCs) as sensors for Point-of Care-Testing (PoCT) applications. We developed a microfluidic platform for the generation of droplets containing DNA-AgNCs in defined, different chemical environments. The droplets are read out fluorescently at two different emission wavelengths. For the pre-evaluation for the usage of biologically relevant matrices with DNA-AgNCs, the response of two different DNA-AgNCs to a variation in pH and sodium chloride concentration was acquired. Our compact and simple setup can detect DNA-AgNCs well below 100 nM and allows the characterization of the fluorescence response of DNA-based biohybrid nanosensors to changes in the chemical environment within short measurement times. The model DNA-AgNCs remain fluorescent throughout the physiologically relevant chloride concentrations and up to 150 mM. Upon shifts in pH, the DNA-AgNCs showed a complex fluorescence intensity response. The model DNA-AgNCs differ strongly in their response characteristics to the applied changes in their environments. With our work, we show the feasibility of the use of DNA-AgNCs as sensors in a simple microfluidic setup that can be used as a building block for PoCT applications while highlighting challenges in their adaption for use with biologically relevant matrices.
Keywords: DNA-templated silver nanoclusters; Point-of-CareTesting; biohybrid nanosensors; fluorescence; microfluidics; nanoclusters; segmented flow.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Solid-State Nanopore Analysis of DNA-Templated Silver Nanoclusters: Voltage-Dependent Translocation and Electrolyte Stability.ACS Appl Mater Interfaces. 2025 Jul 9;17(27):39407-39419. doi: 10.1021/acsami.5c02405. Epub 2025 May 27. ACS Appl Mater Interfaces. 2025. PMID: 40420685 Free PMC article.
-
Plasmon-emitter coupling in cytosine-rich hairpin DNA-templated silver nanoclusters: Thermal reversibility, white light emission, and dynamics inside live cells.J Chem Phys. 2024 Apr 21;160(15):154303. doi: 10.1063/5.0200544. J Chem Phys. 2024. PMID: 38624117
-
A Ratiometric Fluorescence Probe Based on Silver Nanoclusters and CdSe/ZnS Quantum dots for the Detection of Hydrogen Peroxide by Aggregation and Etching.J Fluoresc. 2025 Jun;35(6):3967-3979. doi: 10.1007/s10895-024-03774-x. Epub 2024 Jun 22. J Fluoresc. 2025. PMID: 38907118
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Strategies of testing for syphilis during pregnancy.Cochrane Database Syst Rev. 2014 Oct 29;2014(10):CD010385. doi: 10.1002/14651858.CD010385.pub2. Cochrane Database Syst Rev. 2014. PMID: 25352226 Free PMC article.
References
-
- Drain P.K., Dorward J., Violette L.R., Quame-Amaglo J., Thomas K.K., Samsunder N., Ngobese H., Mlisana K., Moodley P., Donnell D., et al. Point-of-Care HIV Viral Load Testing Combined with Task Shifting to Improve Treatment Outcomes (STREAM): Findings from an Open-Label, Non-Inferiority, Randomised Controlled Trial. Lancet HIV. 2020;7:e229–e237. doi: 10.1016/S2352-3018(19)30402-3. - DOI - PMC - PubMed
-
- Hinson J.S., Rothman R.E., Carroll K., Mostafa H.H., Ghobadi K., Smith A., Martinez D., Shaw-Saliba K., Klein E., Levin S. Targeted Rapid Testing for SARS-CoV-2 in the Emergency Department Is Associated with Large Reductions in Uninfected Patient Exposure Time. J. Hosp. Infect. 2021;107:35–39. doi: 10.1016/j.jhin.2020.09.035. - DOI - PMC - PubMed
-
- Dinnes J., Sharma P., Berhane S., Van Wyk S.S., Nyaaba N., Domen J., Taylor M., Cunningham J., Davenport C., Dittrich S., et al. Rapid, Point-of-Care Antigen Tests for Diagnosis of SARS-CoV-2 Infection. Cochrane Database Syst. Rev. 2022;7:CD013705. doi: 10.1002/14651858.CD013705.pub3. - DOI - PMC - PubMed
-
- Brendish N.J., Poole S., Naidu V.V., Mansbridge C.T., Norton N.J., Wheeler H., Presland L., Kidd S., Cortes N.J., Borca F., et al. Clinical Impact of Molecular Point-of-Care Testing for Suspected COVID-19 in Hospital (COV-19POC): A Prospective, Interventional, Non-Randomised, Controlled Study. Lancet Respir. Med. 2020;8:1192–1200. doi: 10.1016/S2213-2600(20)30454-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

