Bioluminescence in Clinical and Point-of-Care Testing
- PMID: 40710072
- PMCID: PMC12294009
- DOI: 10.3390/bios15070422
Bioluminescence in Clinical and Point-of-Care Testing
Abstract
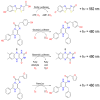
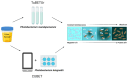
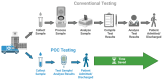
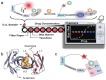
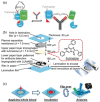
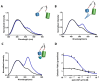
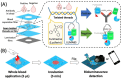
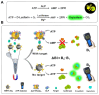
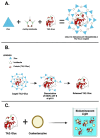
Point-of-care testing (POCT) offers a transformative approach to diagnostics by enabling rapid and accurate results at or near the site of patient care. This is especially valuable in critical care, emergency settings, and resource-limited areas. However, one major limitation of POCT remains its analytical sensitivity, particularly in detecting low concentrations of analytes. To address this, various innovations are being explored, including advanced sensors, signal amplification, and sensitive labels. Among these, bioluminescent proteins have gained attention for their high sensitivity, fast readout, minimal background interference, and simplified instrumentation. Bioluminescence-light emission from biochemical reactions-presents an ideal platform for enhancing POCT sensitivity. In parallel, metal-organic frameworks (MOFs), especially structures like ZIF-8, are emerging as valuable materials in biosensing. Their high porosity, tunable surface properties, and ability to host biomolecules make them excellent candidates for improving analyte capture and signal transduction. When integrated with bioluminescent systems, MOFs can stabilize proteins, concentrate targets, and enhance overall assay performance. This review highlights the role of bioluminescent proteins in medical diagnostics and their application in POCT platforms. We also discuss the potential synergy between MOFs and bioluminescence to overcome current sensitivity limitations. Finally, we examine existing challenges and strategies to optimize these technologies for robust, field-deployable diagnostic tools. By leveraging both the natural sensitivity of bioluminescence and the structural advantages of MOFs, next-generation POCT systems can achieve superior performance, driving forward diagnostic accessibility and patient care outcomes.
Keywords: bioluminescence; clinical diagnostics; encapsulation; metal organic frameworks; point-of-care.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Opportunities in functionalized metal-organic frameworks (MOFs) with open metal sites for optical biosensor application.Adv Colloid Interface Sci. 2025 Oct;344:103598. doi: 10.1016/j.cis.2025.103598. Epub 2025 Jul 7. Adv Colloid Interface Sci. 2025. PMID: 40651338 Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

