Electrochemical Impedance Spectroscopy-Based Biosensors for Label-Free Detection of Pathogens
- PMID: 40710093
- PMCID: PMC12293632
- DOI: 10.3390/bios15070443
Electrochemical Impedance Spectroscopy-Based Biosensors for Label-Free Detection of Pathogens
Abstract
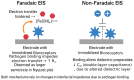
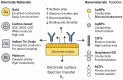
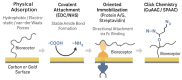
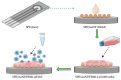
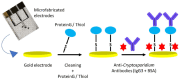
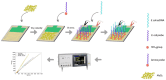
The escalating threat of infectious diseases necessitates the development of diagnostic technologies that are not only rapid and sensitive but also deployable at the point of care. Electrochemical impedance spectroscopy (EIS) has emerged as a leading technique for the label-free detection of pathogens, offering a unique combination of sensitivity, non-invasiveness, and adaptability. This review provides a comprehensive overview of the design and application of EIS-based biosensors tailored for pathogen detection, focusing on critical components such as biorecognition elements, electrode materials, nanomaterial integration, and surface immobilization strategies. Special emphasis is placed on the mechanisms of signal generation under Faradaic and non-Faradaic modes and how these underpin performance characteristics such as the limit of detection, specificity, and response time. The application spectrum spans bacterial, viral, fungal, and parasitic pathogens, with case studies highlighting detection in complex matrices such as blood, saliva, food, and environmental water. Furthermore, integration with microfluidics and point-of-care systems is explored as a pathway toward real-world deployment. Emerging strategies for multiplexed detection and the utilization of novel nanomaterials underscore the dynamic evolution of the field. Key challenges-including non-specific binding, matrix effects, the inherently low ΔRct/decade sensitivity of impedance transduction, and long-term stability-are critically evaluated alongside recent breakthroughs. This synthesis aims to support the future development of robust, scalable, and user-friendly EIS-based pathogen biosensors with the potential to transform diagnostics across healthcare, food safety, and environmental monitoring.
Keywords: biorecognition strategies; microfluidic integration; nanomaterial enhancement; non-faradaic detection; point-of-care diagnostics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Optical and Electrochemical Biosensors for Detection of Pathogens Using Metal Nanoclusters: A Systematic Review.Biosensors (Basel). 2025 Jul 17;15(7):460. doi: 10.3390/bios15070460. Biosensors (Basel). 2025. PMID: 40710110 Free PMC article. Review.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Zhao Y.-X., Shaw P., Wen X., Dou Q., Zhang X. Recent Developments towards Portable Point-of-Care Diagnostic Devices for Pathogen Detection. Sens. Diagn. 2022;1:252–268. doi: 10.1039/d1sd00017a. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

