Sensitivity and Cross-Reactivity Analysis of Serotype-Specific Anti-NS1 Serological Assays for Dengue Virus Using Optical Modulation Biosensing
- PMID: 40710103
- PMCID: PMC12293919
- DOI: 10.3390/bios15070453
Sensitivity and Cross-Reactivity Analysis of Serotype-Specific Anti-NS1 Serological Assays for Dengue Virus Using Optical Modulation Biosensing
Abstract
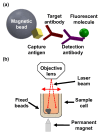
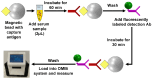
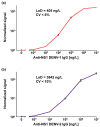
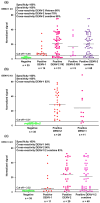
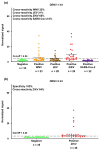
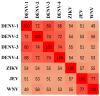
Dengue virus (DENV) poses a major global health concern, with over 6.5 million cases and 7300 deaths reported in 2023. Accurate serological assays are essential for tracking infection history, evaluating disease severity, and guiding vaccination strategies. However, existing assays are limited in their specificity, sensitivity, and cross-reactivity. Using optical modulation biosensing (OMB) technology and non-structural protein 1 (NS1) antigens from DENV-1-3, we developed highly sensitive and quantitative serotype-specific anti-DENV NS1 IgG serological assays. The OMB-based assays offered a wide dynamic range (~4-log), low detection limits (~400 ng/L), fast turnaround (1.5 h), and a simplified workflow. Using samples from endemic (Vietnam) and non-endemic (Israel) regions, we assessed intra-DENV and inter-Flavivirus cross-reactivity. Each assay detected DENV infection with a 100% sensitivity for the corresponding serotype and 64% to 90% for other serotypes. Cross-reactivity with Zika, Japanese encephalitis, and West Nile viruses ranged from 21% to 65%, reflecting NS1 antigen conservation. Our study provides valuable insights into the cross-reactivity of DENV NS1 antigens widely used in research and highlights the potential of OMB-based assays for quantitative and epidemiological studies. Ongoing efforts should aim to minimize cross-reactivity while maintaining sensitivity and explore integration with complementary platforms for improved diagnostic precision.
Keywords: Japanese encephalitis virus; West Nile virus; cross-reactivity; dengue virus; non-structural protein 1; optical detection; serology; zika virus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- WHO Dengue and Severe Dengue. World Helath Organization. [(accessed on 9 July 2025)]. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
-
- Jentes E.S., Lash R.R., Johansson M.A., Sharp T.M., Henry R., Brady O.J., Sotir M.J., Hay S.I., Margolis H.S., Brunette G.W. Evidence-based risk assessment and communication: A new global dengue-risk map for travellers and clinicians. J. Travel. Med. 2016;23:6. doi: 10.1093/jtm/taw062. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

