An Automated Microfluidic Platform for In Vitro Raman Analysis of Living Cells
- PMID: 40710109
- PMCID: PMC12293637
- DOI: 10.3390/bios15070459
An Automated Microfluidic Platform for In Vitro Raman Analysis of Living Cells
Abstract
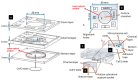
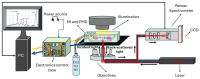
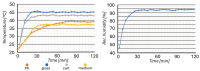
We present a miniaturized, inexpensive, and user-friendly microfluidic platform to support biological applications. The system integrates a mini-incubator providing controlled environmental conditions and housing a microfluidic device for long-term cell culture experiments. The incubator is designed to be compatible with standard inverted optical microscopes and Raman spectrometers, allowing for the non-invasive imaging and spectroscopic analysis of cell cultures in vitro. The microfluidic device, which reproduces a dynamic environment, was optimized to sustain a passive, gravity-driven flow of medium, eliminating the need for an external pumping system and reducing mechanical stress on the cells. The platform was tested using Raman analysis and adherent tumoral cells to assess proliferation prior and subsequent to hydrogen peroxide treatment for oxidative stress induction. The results demonstrated a successful adhesion of cells onto the substrate and their proliferation. Furthermore, the platform is suitable for carrying out optical monitoring of cultures and Raman analysis. In fact, it was possible to discriminate spectra deriving from control and hydrogen peroxide-treated cells in terms of DNA backbone and cellular membrane modification effects provoked by reactive oxygen species (ROS) activity. The 800-1100 cm-1 band highlights the destructive effects of ROS on the DNA backbone's structure, as its rupture modifies its vibration; moreover, unpaired nucleotides are increased in treated sample, as shown in the 1154-1185 cm-1 band. Protein synthesis deterioration, led by DNA structure damage, is highlighted in the 1257-1341 cm-1, 1440-1450 cm-1, and 1640-1670 cm-1 bands. Furthermore, membrane damage is emphasized in changes in the 1270, 1301, and 1738 cm-1 frequencies, as phospholipid synthesis is accelerated in an attempt to compensate for the membrane damage brought about by the ROS attack. This study highlights the potential use of this platform as an alternative to conventional culturing and analysis procedures, considering that cell culturing, optical imaging, and Raman spectroscopy can be performed simultaneously on living cells with minimal cellular stress and without the need for labeling or fixation.
Keywords: Raman spectroscopy; cancer cells; cell culturing; in vitro culturing; microfluidic screening devices; microfluidics; mini-incubator; optical imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ramaiahgari S.C., den Braver M.W., Herpers B., Terpstra V., Commandeur J.N., van de Water B., Price L.S. A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Arch. Toxicol. 2014;88:1083–1095. doi: 10.1007/s00204-014-1215-9. - DOI - PubMed
-
- de Siqueira L., Grenho L., Fernandes M.H., Monteiro F.J., Trichês E.S. 45S5 bioglass-derived glass-ceramic scaffolds containing niobium obtained by gelcasting method. Mater. Res. 2021;24:e20200403. doi: 10.1590/1980-5373-mr-2020-0403. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

