Graphene-Bacteriophage Hybrid Nanomaterials for Specific and Rapid Electrochemical Detection of Pathogenic Bacteria
- PMID: 40710116
- PMCID: PMC12294051
- DOI: 10.3390/bios15070467
Graphene-Bacteriophage Hybrid Nanomaterials for Specific and Rapid Electrochemical Detection of Pathogenic Bacteria
Abstract
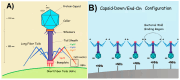
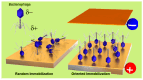
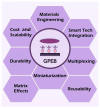
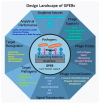
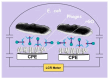
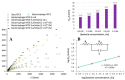
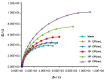
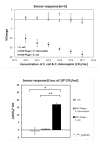
Efficient and rapid detection of bacterial pathogens is crucial for food safety and effective disease control. While conventional methods such as PCR and ELISA are accurate, they are time-consuming, costly, and often require specialized infrastructure. Recently, electrochemical biosensors integrating graphene nanomaterials with bacteriophages-termed graphages-have emerged as promising platforms for pathogen detection, offering fast, specific, and highly responsive detection. This review critically examines all electrochemical biosensors reported to date that utilize graphene-phage hybrids. Key aspects addressed include the types of graphene nanomaterials and bacteriophages used, immobilization strategies, electrochemical transduction mechanisms, and sensor metrics-such as detection limits, linear ranges, and ability to perform in real matrices. Particular attention is given to the role of phage orientation, surface functionalization, and the use of receptor binding proteins. Finally, current limitations and opportunities for future research are outlined, including prospects for genetic engineering and sensor miniaturization. This review serves as a comprehensive reference for researchers developing phage-based biosensors, especially those interested in integrating carbon nanomaterials for improved electroanalytical performance.
Keywords: bacteriophages; bioreceptor immobilization; electrochemical biosensors; graphene nanomaterials; nanomaterial–bioreceptor interfaces; pathogen detection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


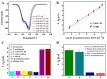
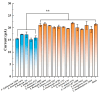
References
-
- Oliver H.F., Wiedmann M., Boor K.J. Environmental Reservoir and Transmission into the Mammalian Host. In: Goldfine H., Shen H., editors. Listeria Monocytogenes: Pathogenesis and Host Response. 1st ed. Springer; Boston, MA, USA: 2007. pp. 111–138. - DOI
-
- Havelaar A.H., Kirk M.D., Torgerson P.R., Gibb H.J., Hald T., Lake R.J., Praet N., Bellinger D.C., De Silva N.R., Gargouri N., et al. World Health Organization Global Estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12:e1001923. doi: 10.1371/journal.pmed.1001923. - DOI - PMC - PubMed
-
- Jaffee S., Henson S., Unnevehr L., Grace D., Cassou E. The Safe Food Imperative: Accelerating Progress in Low- and Middle-Income Countries. World Bank; Washington, DC, USA: 2019. (Agriculture and Food Series).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

