Hypergravity and ERK Inhibition Combined Synergistically Reduce Pathological Tau Phosphorylation in a Neurodegenerative Cell Model
- PMID: 40710311
- PMCID: PMC12293720
- DOI: 10.3390/cells14141058
Hypergravity and ERK Inhibition Combined Synergistically Reduce Pathological Tau Phosphorylation in a Neurodegenerative Cell Model
Abstract
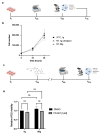
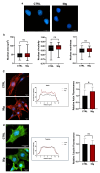
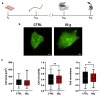
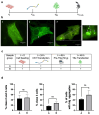
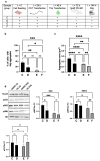
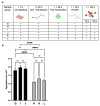
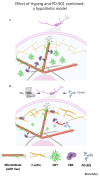
This study evaluates the effects of hypergravity (HG) on a neurodegenerative model in vitro, looking at how HG influences Tau protein aggregation in Mouse Hippocampal Neuronal Cells (HT22) induced by neurofibrillary tangle seeds. Overall, 50× g significantly, synergistically, reduced the Tau aggregate Area when combined with ERK-inhibitor PD-0325901, correlating with decreased phosphorylation at critical residues pS262 and pS396. These findings suggest HG treatments may help mitigate cytoskeletal damage linked to Tau aggregation.
Keywords: European Space Agency (ESA); PD-0325901; hypergravity; large diameter centrifuge (LDC); neurodegeneration; tauopathies.
Conflict of interest statement
Author Jack J.W.A. Van Loon was employed by the European Space Agency. The remaining authors, Valerio Mignucci, Ivana Barravecchia, Davide De Luca, Giacomo Siano, Cristina Di Primio, and Debora Angeloni, declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Activation of Ras-ERK Signaling and GSK-3 by Amyloid Precursor Protein and Amyloid Beta Facilitates Neurodegeneration in Alzheimer's Disease.eNeuro. 2017 Mar 27;4(2):ENEURO.0149-16.2017. doi: 10.1523/ENEURO.0149-16.2017. eCollection 2017 Mar-Apr. eNeuro. 2017. PMID: 28374012 Free PMC article.
-
hiPSC-neurons recapitulate the subtype-specific cell intrinsic nature of susceptibility to neurodegenerative disease-relevant aggregation.Acta Neuropathol Commun. 2025 May 19;13(1):108. doi: 10.1186/s40478-025-02000-4. Acta Neuropathol Commun. 2025. PMID: 40390134 Free PMC article.
-
Inhibition of Bone Morphogenetic Protein Signaling Prevents Tau Pathology in iPSC Derived Neurons and PS19 Mice.Ann Neurol. 2025 Apr;97(4):657-672. doi: 10.1002/ana.27149. Epub 2024 Dec 7. Ann Neurol. 2025. PMID: 39644182 Free PMC article.
-
Tauopathies: Emerging discoveries on tau protein, with a special focus on Alzheimer's disease.Neuropeptides. 2025 Aug;112:102536. doi: 10.1016/j.npep.2025.102536. Epub 2025 Jul 2. Neuropeptides. 2025. PMID: 40628019 Review.
-
Tau protein aggregation: A therapeutic target for neurodegenerative diseases.Adv Protein Chem Struct Biol. 2025;146:77-136. doi: 10.1016/bs.apcsb.2024.11.012. Epub 2025 May 12. Adv Protein Chem Struct Biol. 2025. PMID: 40610078 Review.
References
-
- Clément G., Wood S.J., Reschke M.F. Effects of microgravity on the interaction of vestibular and optokinetic nystagmus in the vertical plane. Aviat. Space Environ. Med. 1992;63:778–784. - PubMed
-
- Acharya M.M., Baulch J.E., Klein P.M., Baddour A.A.D., Apodaca L.A., Kramár E.A., Alikhani L., Garcia C., Angulo M.C., Batra R.S., et al. New Concerns for Neurocognitive Function during Deep Space Exposures to Chronic, Low Dose-Rate, Neutron Radiation. eNeuro. 2019;6:ENEURO.0094-19.2019. doi: 10.1523/ENEURO.0094-19.2019. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

