Influence of Hypoxia on Tumor Heterogeneity, DNA Repair, and Cancer Therapy: From Molecular Insights to Therapeutic Strategies
- PMID: 40710312
- PMCID: PMC12294107
- DOI: 10.3390/cells14141057
Influence of Hypoxia on Tumor Heterogeneity, DNA Repair, and Cancer Therapy: From Molecular Insights to Therapeutic Strategies
Abstract
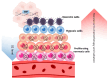
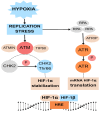
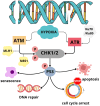
Hypoxia, characterized by a reduction in tissue oxygen levels, is a hallmark of many solid tumors and affects a range of cellular processes, including DNA repair. In low-oxygen conditions, cancer cells often suppress key DNA repair pathways such as homologous recombination (HR), leading to the accumulation of DNA damage and increased genomic instability. These changes not only drive tumor progression but also contribute to resistance against conventional therapies. Hypoxia significantly reduces the effectiveness of oxygen-dependent treatments, including radiotherapy and many chemotherapeutic agents. To address this limitation, bioreductive drugs have been developed that become selectively activated in hypoxic environments, providing targeted cytotoxic effects within oxygen-deprived tumor regions. Additionally, the rapid growth of tumors often results in disorganized and inefficient vasculature, further impairing the delivery of oxygen and therapeutic agents. This review explores the molecular mechanisms by which hypoxia disrupts DNA repair and contributes to treatment resistance. It also presents emerging therapeutic strategies aimed at targeting the hypoxic tumor microenvironment to improve treatment efficacy and patient outcomes.
Keywords: DNA repair; cancer; cancer therapy; hypoxia; hypoxia-inducible factors (HIFs); hypoxia-targeted therapy; therapeutic resistance; tumor heterogeneity; tumor microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Oncolytic reovirus enhances the effect of CEA immunotherapy when combined with PD1-PDL1 inhibitor in a colorectal cancer model.Immunotherapy. 2025 Apr;17(6):425-435. doi: 10.1080/1750743X.2025.2501926. Epub 2025 May 12. Immunotherapy. 2025. PMID: 40353308
-
The Molecular Mechanisms of Actions, Effects, and Clinical Implications of PARP Inhibitors in Epithelial Ovarian Cancers: A Systematic Review.Int J Mol Sci. 2022 Jul 23;23(15):8125. doi: 10.3390/ijms23158125. Int J Mol Sci. 2022. PMID: 35897700 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
Cited by
-
Predicting the therapeutic efficacy of uterine artery embolization for adenomyosis using a combined model based on MRI radiomics and clinical characteristics.Abdom Radiol (NY). 2025 Aug 18. doi: 10.1007/s00261-025-05176-4. Online ahead of print. Abdom Radiol (NY). 2025. PMID: 40824539
References
-
- Bigos K.J., Quiles C.G., Lunj S., Smith D.J., Krause M., Troost E.G., West C.M., Hoskin P., Choudhury A. Tumour Response to Hypoxia: Understanding the Hypoxic Tumour Microenvironment to Improve Treatment Outcome in Solid Tumours. Front. Oncol. 2024;14:1331355. doi: 10.3389/fonc.2024.1331355. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

