The Role of Neurohypophysial Hormones in the Endocrine and Paracrine Control of Gametogenesis in Fish
- PMID: 40710314
- PMCID: PMC12293452
- DOI: 10.3390/cells14141061
The Role of Neurohypophysial Hormones in the Endocrine and Paracrine Control of Gametogenesis in Fish
Abstract
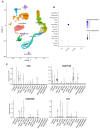
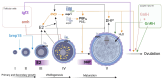
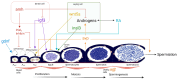
Arginine vasopressin (AVP) and oxytocin (OXT) are neuropeptides traditionally recognized for their roles in the control of osmoregulation, blood pressure, lactation, and parturition in mammals. However, growing evidence suggests that AVPand OXT also regulate gonadal functions in teleost fish. Their expression in both male and female gonads, the presence of their receptors in ovaries and testes, and their interactions with steroids and other gonadal factors indicate a role in modulating gametogenesis and steroidogenesis via autocrine and paracrine mechanisms. Here, we review the current findings on AVP and OXT in teleost gonads, compared to the observed functions in mammals, emphasizing their systemic interactions within the hypothalamic-pituitary-gonadal (HPG) axis. While highlighting the roles of gonadal AVP and OXT in fish reproduction, we underscore the need for further research to unravel their complex multifactorial regulatory networks. Insights into the vasopressinergic system could enhance aquaculture practices by improving spawning success and reproductive efficiency.
Keywords: fish endocrinology; isotocin; oogenesis; paracrine regulation; spermatogenesis; steroidogenesis; vasotocin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Revisiting the specific and potentially independent role of the gonad in hormone regulation and reproductive behavior.J Exp Biol. 2024 Nov 1;227(21):jeb247686. doi: 10.1242/jeb.247686. Epub 2024 Nov 7. J Exp Biol. 2024. PMID: 39508240 Review.
-
Reproductive roles of the vasopressin/oxytocin neuropeptide family in teleost fishes.Front Endocrinol (Lausanne). 2022 Oct 13;13:1005863. doi: 10.3389/fendo.2022.1005863. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36313759 Free PMC article. Review.
-
Asprosin modulates female reproductive functions in teleosts: An in vitro study in Channa punctata.Gen Comp Endocrinol. 2025 Jul;370:114770. doi: 10.1016/j.ygcen.2025.114770. Epub 2025 Jun 11. Gen Comp Endocrinol. 2025. PMID: 40513993
-
Independent contribution of gonads and sex chromosomes to sex differences in bone mass and strength in the four-core genotypes mouse model.J Bone Miner Res. 2024 Oct 29;39(11):1659-1672. doi: 10.1093/jbmr/zjae147. J Bone Miner Res. 2024. PMID: 39255371
-
Deciphering gonadal transcriptome reveals circRNA-miRNA-mRNA regulatory network involved in sex differentiation and gametogenesis of Apostichopus japonicus.Mol Genet Genomics. 2025 Jul 19;300(1):70. doi: 10.1007/s00438-025-02276-0. Mol Genet Genomics. 2025. PMID: 40682658
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

