Temporal and Severity-Dependent Alterations in Plasma Extracellular Vesicle Profiles Following Spinal Cord Injury
- PMID: 40710318
- PMCID: PMC12294027
- DOI: 10.3390/cells14141065
Temporal and Severity-Dependent Alterations in Plasma Extracellular Vesicle Profiles Following Spinal Cord Injury
Abstract
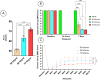
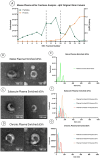
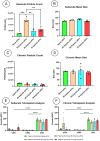
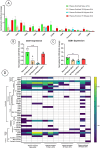
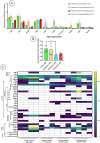
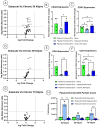
Spinal cord injury (SCI) triggers both local and systemic pathological responses that evolve over time and differ with injury severity. Small extracellular vesicles (sEVs), known mediators of intercellular communication, may serve as biomarkers reflecting these complex dynamics. In this study, we investigated whether SCI severity modulates the composition and abundance of circulating plasma-derived sEVs across subacute and chronic phases. Using a graded thoracic contusion model in mice, plasma was collected at defined timepoints post-injury. sEVs were isolated via size-exclusion chromatography and characterized using nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), and MACSPlex surface marker profiling. We observed an SCI-dependent increase in sEVs during the subacute (7 days) phase, most notably in moderate injuries (50 kdyne), with overall vesicle counts lower chronically (3 months). CD9 emerged as the predominant tetraspanin sEV marker, while CD63 and CD81 were generally present at low levels across all injury severities and timepoints. Surface sEV analysis revealed dynamic regulation of CD41+, CD44+, and CD61+ in the CD9+ sEV subset, suggesting persistent systemic signaling activity. These markers, traditionally associated with platelet function, may also reflect immune or reparative responses following SCI. Our findings highlight the evolving nature of sEV profiles after SCI and support their potential as non-invasive biomarkers for monitoring injury progression.
Keywords: chronic phase; injury severity; plasma biomarkers; platelet activation; small extracellular vesicles (sEVs); spinal cord injury (SCI); subacute phase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Single vesicle analysis reveals the release of tetraspanin positive extracellular vesicles into circulation with high intensity intermittent exercise.J Physiol. 2023 Nov;601(22):5093-5106. doi: 10.1113/JP284047. Epub 2023 Mar 16. J Physiol. 2023. PMID: 36855276 Free PMC article.
-
Engineered small extracellular vesicles for targeted delivery of perlecan to stabilise the blood-spinal cord barrier after spinal cord injury.Clin Transl Med. 2025 Jun;15(6):e70381. doi: 10.1002/ctm2.70381. Clin Transl Med. 2025. PMID: 40538064 Free PMC article.
-
Human Infrapatellar Fat Pad Mesenchymal Stem Cell-derived Extracellular Vesicles Purified by Anion Exchange Chromatography Suppress Osteoarthritis Progression in a Mouse Model.Clin Orthop Relat Res. 2024 Jul 1;482(7):1246-1262. doi: 10.1097/CORR.0000000000003067. Epub 2024 Apr 19. Clin Orthop Relat Res. 2024. PMID: 38662932 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Spinal Cord Injury. [(accessed on 30 May 2025)]. Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

