Assessment of Feasibility of the M2 Macrophage-Based Adoptive Gene Transfer Strategy for Osteoarthritis with a Mouse Model
- PMID: 40710320
- PMCID: PMC12293217
- DOI: 10.3390/cells14141067
Assessment of Feasibility of the M2 Macrophage-Based Adoptive Gene Transfer Strategy for Osteoarthritis with a Mouse Model
Abstract
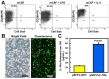
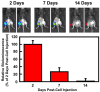
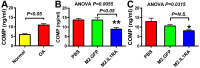
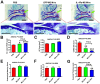
Current osteoarthritis (OA) therapies fail to yield long-term clinical benefits, due in part to the lack of a mechanism for the targeted and confined delivery of therapeutics to OA joints. This study evaluates if M2 macrophages are effective cell vehicles for the targeted and confined delivery of therapeutic genes to OA joints. CT bioluminescence in vivo cell tracing and fluorescent microscopy reveal that intraarticularly injected M2 macrophages were recruited to and retained at inflamed synovia. The feasibility of an M2 macrophage-based adoptive gene transfer strategy for OA was assessed using IL-1Ra as the therapeutic gene in a mouse tibial plateau injury model. Mouse M2 macrophages were transduced with lentiviral vectors expressing IL-1Ra or GFP. The transduced macrophages were intraarticularly injected into injured joints at 7 days post-injury and OA progression was monitored with plasma COMP and histology at 4 weeks. The IL-1Ra-expressing M2 macrophage treatment reduced plasma COMP, increased the area and width of the articular cartilage layer, decreased synovium thickness, and reduced the OARSI OA score without affecting the osteophyte maturity and meniscus scores when compared to the GFP-expressing M2 macrophage-treated or PBS-treated controls. When the treatment was given at 5 weeks post-injury, at which time OA should have developed, the IL-1Ra-M2 macrophage treatment also reduced plasma COMP, had a greater articular cartilage area and width, decreased synovial thickness, and reduced the OARSI OA score without an effect on the meniscus and osteophyte maturity scores at 8 weeks post-injury. In conclusion, the IL-1Ra-M2 macrophage treatment, given before or after OA was developed, delayed OA progression, indicating that the M2 macrophage-based adoptive gene transfer strategy for OA is tenable.
Keywords: IL-1Ra; adoptive gene transfer; inflammation; macrophages; osteoarthritis; treatment.
Conflict of interest statement
All authors (Sheng, M.H.-C., Baylink, D.J., Rundle, C.H. and Lau, K.-H.W.) state that they have no conflicts of interest to declare. The views, opinions, and/or findings contained in this report are those of the authors and should not be construed as an official position, policy, or decision of the US Army, the US Department of Veterans’ Affairs, or the United States government, unless so designated by other documentation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

